Abstract
Objective
Toll-like receptors (TLRs) that mediate inflammatory responses play an important role in epilepsy; however, whether TLR1 is also involved in epileptogenesis remains unclear. Thus, in this study, we investigated the extent and pattern of TLR1 expression in epileptic tissues.
Methods
One-hundred and thirty-two mice were intra-cerebroventricularly injected with PBS or kainic acid (KA) and were examined at 1, 3, 8 and 24 h. The expression pattern and distribution of TLR1 were examined by reverse-transcriptase polymerase chain reaction (RT-PCR), western blot analysis and immunohistochemistry staining.
Results
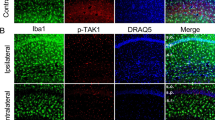
The mRNA and protein levels of TLR1 were significantly upregulated in the hippocampus and temporal cortex of epileptic mice compared with those of controls. TLR1 expression was increased as early as 1 h following KA treatment and peaked at 8 and 24 h. Immunohistochemistry staining demonstrated that TLR1 was distributed in the CA1-3, dentate gyrus and hilus regions of the hippocampus and different cortical regions. Immunofluorescent staining further revealed that TLR1 was primarily expressed in the neurons, microglia, and astrocytes of epileptogenic tissue.
Significance
These results demonstrate that cortical and hippocampal sub-regional expression of TLR1 is altered during epileptogenesis in a time- and location-specific manner, suggesting a close association with the process of epilepsy.





Similar content being viewed by others
References
Ryan K, Liang LP, Rivard C, Patel M. Temporal and spatial increase of reactive nitrogen species in the kainate model of temporal lobe epilepsy. Neurobiol Dis. 2014;64:8–15.
Salami P, Levesque M, Benini R, Behr C, Gotman J, Avoli M. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis 2014.
Maroso MB, Ravizza S, Liu T, Aronica J, Iyer EA, Rossetti M, Molteni C, Casalgrandi M, Manfredi M, Bianchi M. Vezzani A Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–9.
Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40.
He JJ, Li S, Shu HF, Yu SX, Liu SY, Yin Q, et al. The interleukin 17 system in cortical lesions in focal cortical dysplasias. J Neuropathol Exp Neurol. 2013;72:152–63.
Shu HF, Zhang CQ, Yin Q, An N, Liu SY, Yang H. Expression of the interleukin 6 system in cortical lesions from patients with tuberous sclerosis complex and focal cortical dysplasia type IIb. J Neuropathol Exp Neurol. 2010;69:838–49.
Guo W, Zheng DH, Sun FJ, Yang JY, Zang ZL, Liu SY, et al. Expression and cellular distribution of the interleukin 2 signaling system in cortical lesions from patients with focal cortical dysplasia. J Neuropathol Exp Neurol. 2014;73:206–22.
Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131:3256–65.
Iori VM, Rizzi M, Iyer M, Vertemara AM, Carli R, Agresti M, Antonelli A, Bianchi A, Aronica ME, Ravizza E, Vezzani TA. Receptor for Advanced Glycation Endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013;58:102–14.
Hong JC, Kwak IH, Suh KI, Seo EC, Min J, Choi HJ, Kim SY, Park CH, Jo SH, Lee EK, Lee S. Lee KE Microglial Toll-like receptor 2 contributes to kainic acid-induced glial activation and hippocampal neuronal cell death. J Biol Chem. 2010;285:39447–57.
Liu S, Lu L, Cheng X, Xu G, Yang H. Viral infection and focal cortical dysplasia. Ann Neurol 2013.
Zurolo EI, Maroso A, Carbonell M, Anink C, Ravizza JJ, Fluiter T, Spliet K, van Rijen WG, Vezzani PC. Aronica AE Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134:1015–32.
Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–4.
Stridh LS, Naylor PL, Wang AS. Mallard XC Regulation of toll-like receptor 1 and -2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8:45.
K.B.J. Franklin GP. The mouse brain in stereotaxic coordinates. 2edn, Academic Press, San Diego 2001.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94.
Friedman ADR. Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia. 2011;52:33–9.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Kaul D, Habbel P, Derkow K, Kruger C, Franzoni E, Wulczyn FG, et al. Expression of Toll-like receptors in the developing brain. PLoS One. 2012;7:e37767.
Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–81.
Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–9.
Barak B FN, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front Neurosci 2014;272.
Tu XK, Yang WZ, Chen JP, Chen Y, Ouyang LQ, Xu YC, et al. Curcumin inhibits TLR2/4-NF-kappaB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation. 2014;37:1544–51.
Means TK, Golenbock DT, Fenton MJ. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–32.
Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–61.
Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42.
Riban V, Bouilleret V, Pham-Le BT, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112:101–11.
Zhang W, Huguenard JR, Buckmaster PS. Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–96.
Ginsberg SDCS. Expression profile analysis within the human hippocampus: comparison of CA1 and CA3 pyramidal neurons. J Comp Neurol. 2005;487:1419–30.
Jiang C, Schuman EM. Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem Sci. 2002;27:506–13.
Nadler JVPB, Gentry C, Cotman CW. Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J Comp Neurol. 1980;192:333–59.
Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol 2011.
Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58:1701–9.
Cassiani-Ingoni R, Cabral ES, Lunemann JD, Garza Z, Magnus T, Gelderblom H, et al. Borrelia burgdorferi Induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J Neuropathol Exp Neurol. 2006;65:540–8.
Losi G, Cammarota M, Carmignoto G. The role of astroglia in the epileptic brain. Front Pharmacol. 2012;3:132.
Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–202.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81070953, 81071043, 81371424, and 81371430).
Conflict of interest
None of the authors have any conflicts of interest to disclose. We also confirm that we have read the Journal’s position on issues related to ethical publication and affirm that this report is consistent with those guidelines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ji Zhang.
Fa-Xiang Wang and Shi-Yong Liu are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.

11_2015_828_MOESM1_ESM.jpg
Supplement Fig. 1. TLR1 mRNA expression in the hippocampus and temporal cortex following SE. A) Representative RT-PCR results demonstrating TLR1 mRNA expression in the hippocampus of PBS-treated mice and KA-induced mice following 1, 3, 8, and 24 h. The graph indicates that the mean OD value of TLR1 transiently increased from 1 to 24 h after the seizures. TLR1 (relative to GAPDH) mRNA quantification between PBS-treated and seizure mice from 1 to 24 h after seizures is presented. B) Representative band results demonstrating TLR1 mRNA expression in the temporal cortex of PBS-treated mice and KA-induced mice at 1, 3, 8, and 24 h. The graph indicates that the mean OD value of TLR1 transiently increased from 1 to 24 h after seizures. TLR1 (relative to GAPDH) mRNA ratios between PBS-treated and seizure mice at 1 to 24 h after seizures is presented. n = 8 for each subunit, ** P < 0.01 vs PBS group; # P < 0.01 vs KA 8 h group. (JPEG 402 kb)

11_2015_828_MOESM2_ESM.jpg
Supplement Fig. 2. Fluorescent images of TLR1 in the temporal cortex of epileptic mice.A–C) TLR1 was primarily expressed in the cell bodies and dendrites of neurons (A; arrows) and was co-expressed with NeuN (B–C; arrows). D–I) TLR1 staining was also observed in the cytoplasm and end processes of astrocytes (D; arrow) and CD11b-positive microglia (G; arrow). TLR was expressed by GFAP-positive astrocytes (F; arrows) and CD11b-positive microglia (I; arrows). Scale bars = 25 μm. (JPEG 264 kb)
Rights and permissions
About this article
Cite this article
Wang, FX., Liu, SY., Zheng, X. et al. TLR1 expression in mouse brain was increased in a KA-induced seizure model. Inflamm. Res. 64, 487–495 (2015). https://doi.org/10.1007/s00011-015-0828-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0828-7