Abstract
Objective
High-mobility group box-1 (HMGB1) is identified as an extracellularly released mediator of inflammation. In this study, specific monoclonal anti-HMGB1 antibody was administered to rats with acute on chronic liver failure (ACLF) in order to evaluate the therapeutic efficacy of HMGB1 blockade.
Methods
All animals were randomly divided into control group, model group and anti-HMGB1 antibody group. The changes in liver histology and apoptosis of liver tissue were detected by H&E staining and TUNEL assay, respectively. The serum levels of alanine aminotransferase (ALT), endotoxin, HMGB1, tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) were examined. The hepatic levels of HMGB1, caspase3, Toll-like receptor 4 (TLR4) and p65 subunit of NF-κB (P65) were also determined.
Results
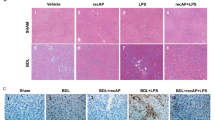
Changes in liver pathology and liver cell apoptosis were greatly attenuated in the anti-HMGB1 antibody group compared with the model group. The serum levels of ALT, endotoxin, TNF-α, IFN-γ and HMGB1 were also decreased in the anti-HMGB1 antibody group. Furthermore, the hepatic levels of HMGB1, TLR4, caspase3 and P65 were also down-regulated by HMGB1 blockade.
Conclusion
Blockade of HMGB1 can confer a protective effect against ACLF in rats, even 24 h after induction of ACLF. The protective effect of HMGB1 blockade is associated with interactions of HMGB1 with the TLR4 signaling pathway and cytokine production.






Similar content being viewed by others
References
Sozinov AS. Systemic endotoxemia during chronic viral hepatitis. Bull Exp Biol Med. 2002;133(2):153–5 (PMID: 12428283).
Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3(2):169–76 (PMID: 12563300).
Cherry S, Silverman N. Host-pathogen interactions in Drosophila: new tricks from an old friend. Nat Immunol. 2006;7(9):911–7 (PMID: 16924255).
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801 (PMID: 16497588).
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8 (PMID: 9851930).
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–42 (PMID: 15803152).
Zhang CC, Krieg S, Shapiro DJ. HMG-1 stimulates estrogen response element binding by estrogen receptor from stably transfected HeLa cells. Mol Endocrinol. 1999;13(4):632–43 (PMID: 10194768).
Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19(8):5237–46 (PMID: 10409715).
Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101(1):296–301 (PMID: 14695889).
Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, Takeyama Y, Kuroda Y. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34(4):487–8 (PMID: 17446855).
Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170(12):1310–6 (PMID: 15374839).
Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, Fukuda T, et al. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008;58(9):2675–85 (PMID: 18759291).
Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178(10):6573–80 (PMID: 17475888).
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–43 (PMID: 15795240).
Wang LW, Wang LK, Chen H, Cheng F, Li X, He CM, Gong ZJ. Ethyl pyruvate protects against experimental acute-on-chronic liver failure in rats. World J Gastroenterol. 2012;18(40):5709–18 (PMID: 23155311).
Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M, Yamada S, Hashimoto S, Fukata S, Abraham E, Maruyama I, Kitajima M, Ishizaka A. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30(9):1755–62 (PMID: 16850155).
Yang R, Miki K, Oksala N, Nakao A, Lindgren L, Killeen ME, Mennander A, Fink MP, Tenhunen J. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R362–9 (PMID: 19494177).
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi. 2006;14(9):643-6. [Chinese (PMID: 16995974)].
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51 (PMID: 10398600).
Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170(7):3890–7 (PMID: 12646658).
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001 (PMID: 12231511).
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–60 (PMID: 14532127).
Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290(4):C990–9 (PMID: 16282196).
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5 (PMID: 12110890).
Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78(1):1–8 (PMID: 15734795).
Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–73 (PMID: 11734424).
Yong-Chen Lu, Yeh Wen-Chen, et al. LPS/TLR4 signal transduction pathway. Cytokines. 2008;42(2):145–51 (PMID: 18304834).
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81071342).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Mauro Teixeira.
Rights and permissions
About this article
Cite this article
Li, X., Wang, LK., Wang, LW. et al. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm. Res. 62, 703–709 (2013). https://doi.org/10.1007/s00011-013-0624-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0624-1