Abstract
Objective
The inflammasome complex involving caspase-1 and nucleotide-binding domain, leucine-rich repeat containing protein (NLRP)3, also known as NALP3 or cryopyrin is important for host responses to microbial pathogens and several autoinflammatory diseases. We investigated the extent to which NLRP3 and caspase-1 control ocular interleukin (IL)-1β production and severity of uveitis (intraocular inflammatory disease) in an established, acute inflammatory uveitis model, endotoxin-induced uveitis (EIU).
Methods
Expression of NLRP3, its adaptor molecule ASC, also known as PYCARD (PYD and CARD domain containing), and caspase-1 were examined by immunoblotting. IL-1β production was measured by enzyme-linked immunosorbent assay (ELISA). Using knockout mice, roles for caspase-1 and NLRP3 were examined in uveitis induced by intraocular injection of Escherichia coli lipopolysaccharide (LPS).
Results
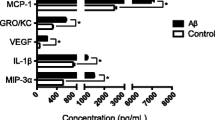
NLRP3, ASC, and caspase-1 proteins are constitutively expressed in eye tissue. During EIU, IL-1β protein production increases; this requires the presence of both caspase-1 and NLRP3. However, severity of EIU is not altered by deficiency in either caspase-1 or NLRP3, as assessed by both intravital microscopy and histology.
Conclusions
These data identify the importance of the NLRP3 inflammasome for IL-1β production in the eye, yet indicate that its participation in EIU is nonessential.




Similar content being viewed by others
References
Martinon F, Mayor A, Tschopp J. The Inflammasomes: Guardians of the Body. Annu Rev Immunol. 2009;27:229–65.
Brodsky IE, Monack D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin Immunol. 2009;21:199–207.
Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35.
Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–9.
Chitkara P, Stojanov S, Kastner DL. The hereditary autoinflammatory syndromes. Pediatr Infect Dis. 2007;26:353–4.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32.
McGarry F, Neilly J, Anderson N, Sturrock R, Field M. A polymorphism within the interleukin 1 receptor antagonist (IL-1Ra) gene is associated with ankylosing spondylitis. Rheumatology. 2001;40:1359–64.
Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–41.
Djouadi K, Nedelec B, Tamouza R, Genin E, Ramasawmy R, Charron D, et al. Interleukin 1 gene cluster polymorphisms in multiplex families with spondylarthropathies. Cytokine. 2001;13:98–103.
Maksymowych WP, Reeve JP, Reveille JD, Akey JM, Buenviaje H, O’Brien L, et al. High-throughput single-nucleotide polymorphism analysis of the IL1RN locus in patients with ankylosing spondylitis by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Arthritis Rheum. 2003;48:2011–8.
Hutyrova B, Pantelidis P, Drabek J, Zurkova M, Kolek V, Lenhart K, et al. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;165:148–51.
Coskun M, Bacanli A, Sallakci N, Alpsoy E, Yavuzer U, Yegin O. Specific interleukin-1 gene polymorphisms in Turkish patients with Behçet’s disease. Exp Dermatol. 2005;14:124–9.
Karasneh J, Hajeer AH, Barrett J, Ollier WE, Thornhill M, Gul A. Association of specific interleukin 1 gene cluster polymorphisms with increased susceptibility for Behcet’s disease. Rheumatology. 2003;42:860–4.
Gonzalez-Benitez JF, Juarez-Verdayes MA, Rodriguez-Martinez S, Cancino-Diaz ME, Garcia-Vasquez F, Cancino-Diaz JC. The NALP3/Cryopyrin-inflammasome complex is expressed in LPS-induced ocular inflammation. Mediators Inflamm 2008;2008:7.
Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–3.
Becker MD, Nobiling R, Planck SR, Rosenbaum JT. Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Methods. 2000;240:23–37.
Rosenzweig HL, Martin TM, Jann MM, Planck SR, Davey MP, Kobayashi K, et al. NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–24.
Rosenzweig H, Galster K, Planck S, Rosenbaum J. NOD1 expression in the eye and functional contribution to IL-1{beta} dependent ocular inflammation in mice. Invest Ophthalmol Vis Sci. 2009;50:1746–53.
Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;11:198–208.
Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kosturan MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74.
Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–15.
Reimer T, Shaw MH, Franchi L, Coban C, Ishii KJ, Akira S, et al. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol. 2010;40:764–9.
Becker MD, O’Rourke LM, Blackman WS, Planck SR, Rosenbaum JT. Reduced leukocyte migration, but normal rolling and arrest, in interleukin-8 receptor homologue knockout mice. Invest Ophthalmol Vis Sci. 2000;41:1812–7.
Tuaillon N, Shen DF, Berger RB, Lu B, Rollins BJ, Chan CC. MCP-1 expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2002;43:1493–8.
Whitcup SM, DeBarge R, Caspi RR, Harning R, Nussenblatt RB, Chan C–C. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993;67:143–50.
Rosenbaum JT, Han YB, Park JM, Kennedy M, Planck SR. TNFα is not a major mediator of endotoxin-induced uveitis: studies in cytokine receptor deficient mice. J Rheumatol. 1998;25:2408–516.
Rosenzweig HL, Martin TM, Planck SR, Galster K, Jann MM, Davey MP, et al. Activation of NOD2 in vivo induces IL-1 beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–36.
Arostegui J, Arnal C, Merino R, Modesto C, Antonia Caballo J, Moreno P, et al. NON2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum. 2007;56:3805–13.
Martin TM, Zhang Z, Kurz P, Rose CD, Chen H, Lu H, et al. The NOD2 defect in Blau syndrome does not result in excess interleukin-1 activity. Arthritis Rheum. 2009;60:611–8.
Brito BE, O’Rourke L, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40:2583–9.
Su SB, Silver PB, Grajewski RS, Agarwal RK, Tang J, Chan CC, et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J Immunol. 2005;175:6303–10.
Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS-S, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1á converting enzyme. Science. 1995;267:2000–3.
Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–23.
Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11.
Shen DF, Buggage RR, Engl HC, Chan CC. Cytokine gene expression in different strains of mice with endotoxin-induced uveitis (EIU). Ocul Immunol Inflamm. 2000;8:221–5.
Imai H, Ohta K, Yoshida A, Suzuki S, Hashizume K, Usami S, et al. {micro}-Crystallin, new candidate protein in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2010;51:3554–9.
Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Optiz B, van der Meer JH, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–35.
Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1β and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–72.
Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91:263–4.
Deal watch: XOMA, Servier to develop anti-IL-1β antibody for inflammatory diseases. Nat Rev Drug Discov 2011;10:166.
Acknowledgments
Thanks are due to Dr. Bruce Magun (Oregon Health and Science University) for the transfer of NLRP3 KO mice. This work was made possible by support from NEI/NIH grants (EY019604, EY019020, and EY010572). The authors are grateful for the support from the Stan and Madelle Rosenfeld Family Trust, the William and Mary Bauman Foundation, the Research to Prevent Blindness Foundation, and the American College of Rheumatology Research and Education Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Graham Wallace.
Rights and permissions
About this article
Cite this article
Rosenzweig, H.L., Woods, A., Clowers, J.S. et al. The NLRP3 inflammasome is active but not essential in endotoxin-induced uveitis. Inflamm. Res. 61, 225–231 (2012). https://doi.org/10.1007/s00011-011-0404-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0404-8