Abstract
Objective
Leukocyte adhesion to vessel walls may initiate vaso-occlusion in sickle cell anemia (SCA); however, the extent to which inflammation participates in this mechanism is not understood. This in vitro study investigated whether inflammatory molecules, commonly augmented in SCA, can affect neutrophil adhesive properties and whether cyclic guanosine monophosphate (cGMP)-elevating agents can inhibit such adhesion.
Subjects and methods
Effects of Interleukin 8 (IL-8), tumor necrosis factor-α (TNF-α), granulocyte macrophage-colony stimulating factor (GM-CSF) cytokines, BAY 73-6691 [phosphodiesterase (PDE)-9A-inhibitor], and BAY 41-2271 (guanylate-cylase stimulator) on the adhesive properties of neutrophils from healthy control (CON) and steady-state SCA individuals were determined using static-adhesion assays.
Results
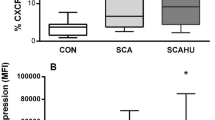
SCA neutrophils demonstrated increased adhesive properties, compared to CON neutrophils; IL-8, TNF-α and GM-CSF increased CON neutrophil adhesion and further increased SCA neutrophil adhesion to fibronectin (FN). The PDE9A inhibitor, BAY-73-6691, significantly reduced basal CON neutrophil and SCA neutrophil adhesion; this was accompanied by decreased SCA neutrophil surface expressions of the L-selectin and CD11b adhesion molecules. BAY-73-6691 also significantly reduced cytokine-stimulated CON neutrophil and SCA neutrophil adhesion to FN; however, this was not accompanied by alterations in adhesion-molecule presentation.
Conclusions
The chronic inflammatory nature of SCA may contribute to leukocyte adhesive functions in SCA. Furthermore, elevation of leukocyte cGMP may be an interesting approach for inhibition of leukocyte adhesion to the vessel wall, even in the presence of inflammatory stimuli.




Similar content being viewed by others
References
Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The cooperative study of sickle cell disease. Blood. 1994;84:643–9.
Kinney TR, Sleeper LA, Wang WC, Zimmerman RA, Pegelow CH, Ohene-Frempong K, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The cooperative study of sickle cell disease. Pediatrics. 1999;103:640–5.
Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, et al. Prediction of adverse outcomes in children with sickle cell disease. N Eng J Med. 2000;342:83–9.
Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009;85:235–42.
Chiang EY, Frenette PS. Sickle cell vaso-occlusion. Hematol Oncol Clin N Am. 2005;19:771–84.
Conran N, Costa FF. Hemoglobin disorders and endothelial cell interactions. Clin Biochem. 2009;42:1824–38.
Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103:2397–400.
Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA. 2002;99:3047–51.
Assis A, Conran N, Canalli AA, Lorand-Metze I, Saad ST, Costa FF. Effect of cytokines and chemokines on sickle neutrophil adhesion to fibronectin. Acta Haematol. 2005;113:130–6.
Canalli AA, Franco-Penteado CF, Traina F, Saad ST, Costa FF, Conran N. Role for cAMP-protein kinase A signalling in augmented neutrophil adhesion and chemotaxis in sickle cell disease. Eur J Haematol. 2007;79:330–7.
Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91:266–74.
Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet–monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24:81–8.
Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–25.
Conran N, Ferreira HH, Lorand-Metze I, Thomazzi SM, Antunes E, de Nucci G. Nitric oxide regulates human eosinophil adhesion mechanisms in vitro by changing integrin expression and activity on the eosinophil cell surface. Br J Pharmacol. 2001;134:632–8.
Conran N, Gambero A, Ferreira HH, Antunes E, de Nucci G. Nitric oxide has a role in regulating VLA-4-integrin expression on the human neutrophil cell surface. Biochem Pharmacol. 2003;66:43–50.
Canalli AA, Franco-Penteado CF, Saad STO, Conran N, Costa FF. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica. 2008;93:605–9.
Mack AK, McGowan Ii VR, Tremonti CK, Ackah D, Barnett C, Machado RF, et al. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: a phase I/II study. Br J Haematol. 2008;142:971–8.
Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009;191:309–39.
Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520.
Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–63.
Almeida CB, Traina F, Lanaro C, Canalli AA, Saad ST, Costa FF, et al. High expression of the cGMP-specific phosphodiesterase, PDE9A, in sickle cell disease (SCD) and the effects of its inhibition in erythroid cells and SCD neutrophils. Br J Haematol. 2008;142:836–44.
Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–5.
Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol. 2005;68:1775–81.
Montes RA, Eckman JR, Hsu LL, Wick TM. Sickle erythrocyte adherence to endothelium at low shear: role of shear stress in propagation of vaso-occlusion. Am J Hematol. 2002;70:216–27.
Rodgers GP, Schechter AN, Noguchi CT, Klein HG, Nienhuis AW, Bonner RF. Periodic microcirculatory flow in patients with sickle-cell disease. N Eng J Med. 1984;311:1534–8.
English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll–Hypaque. J Immunol Methods. 1974;5:249–52.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9.
Benkerrou M, Delarche C, Brahimi L, Fay M, Vilmer E, Elion J, et al. Hydroxyurea corrects the dysregulated l-selectin expression and increased H(2)O(2) production of polymorphonuclear neutrophils from patients with sickle cell anemia. Blood. 2002;99:2297–303.
Finnegan EM, Barabino GA, Liu XD, Chang HY, Jonczyk A, Kaul DK. Small-molecule cyclic alpha V beta 3 antagonists inhibit sickle red cell adhesion to vascular endothelium and vasoocclusion. Am J Physiol Heart Circ Physiol. 2007;293:H1038–45.
Finnegan EM, Turhan A, Golan DE, Barabino GA. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. Am J Hematol. 2007;82:266–75.
Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112:3474–83.
Conran N, Saad ST, Costa FF, Ikuta T. Leukocyte numbers correlate with plasma levels of granulocyte–macrophage colony-stimulating factor in sickle cell disease. Ann Hematol. 2007;86:255–61.
Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol. 1994;87:592–7.
Canalli AA, Proenca-Ferreira R, Franco-Penteado CF, Traina F, Sakamoto TM, Saad ST, et al. Participation of the Mac-1, LFA-1 and VLA-4 integrins in the in vitro adhesion of sickle cell disease neutrophils to endothelial layers, and reversal of adhesion by simvastatin. Haematologica. 2011 [Epub ahead of print].
Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–7.
Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44.
Lum AF, Wun T, Staunton D, Simon SI. Inflammatory potential of neutrophils detected in sickle cell disease. Am J Hematol. 2004;76:126–33.
Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol. 2006;13:40–4.
von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–42.
Smalley DM, Ley K. l-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–66.
Menezes GB, Lee WY, Zhou H, Waterhouse CC, Cara DC, Kubes P. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J Immunol. 2009;183:7557–68.
Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47.
Fagerholm SC, Varis M, Stefanidakis M, Hilden TJ, Gahmberg CG. alpha-chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood. 2006;108:3379–86.
Hogg N, Leitinger B. Shape and shift changes related to the function of leukocyte integrins LFA-1 and Mac-1. J Leukoc Biol. 2001;69:893–8.
Bischoff E, Stasch JP. Effects of the sGC stimulator BAY 41-2272 are not mediated by phosphodiesterase 5 inhibition. Circulation. 2004;110:e320–1. author reply e320-321.
Mullershausen F, Russwurm M, Friebe A, Koesling D. Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41-2272. Circulation. 2004;109:1711–3.
Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–53.
Huang LJ, Yoon MH, Choi JI, Kim WM, Lee HG, Kim YO. Effect of sildenafil on neuropathic pain and hemodynamics in rats. Yonsei Med J. 2010;51:82–7.
Acknowledgments
The authors thank Fernanda Perreira Cunha for assistance with flow cytometry procedures. This study was funded by FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil. The Hematology and Hemotherapy Center, UNICAMP, forms part of the National Institute of Blood, Brazil (INCT de Sangue, CNPq/MCT/FAPESP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Graham Wallace.
Rights and permissions
About this article
Cite this article
Miguel, L.I., Almeida, C.B., Traina, F. et al. Inhibition of phosphodiesterase 9A reduces cytokine-stimulated in vitro adhesion of neutrophils from sickle cell anemia individuals. Inflamm. Res. 60, 633–642 (2011). https://doi.org/10.1007/s00011-011-0315-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0315-8