Abstract
Objective
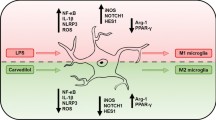
The aim of this paper was to investigate the inhibitory effect of peroxisome proliferator-activated receptor-gamma (PPARγ) agonist pioglitazone on microglia inflammation induced by lipopolysaccharide (LPS).
Materials and methods
Highly aggressively proliferating immortalized cells were used from a rat microglial cell line. Expression of PPARγ, inducible NO synthase (iNOS), the p42/44 extracellular signal-regulated kinase (ERK) MAPKs, c-Jun NH2-terminal kinases (JNKs) and p38 MAPK were determined by Western blot analysis. The protein levels of tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) were determined by enzyme-linked immunosorbent assay. The production of nitric oxide (NO) was determined by a Nitric Oxide Assay Kit. The subcellular localization of PPARγ was studied by immunofluorescence microscopy analysis and nuclear-cytosolic fractionation technology, respectively. The transcriptional activity of PPARγ was detected by PPRE-Luciferase transcription assay.
Results
Pioglitazone effectively inhibited NO, iNOS, TNF-α, IL-6, IL-1β production in LPS-stimulated microglial cells. Additionally, pioglitazone suppressed PPARγ loss; enhanced transcriptional activity of PPARγ; and inhibited nucleus-export of PPARγ in microglia induced by LPS. And p38 MAPK inhibitor SB203580 had the similarity effects with pioglitazone. Signal transduction studies indicated that pioglitazone blocked the phosphorylation of p38 MAPK challenged by LPS.
Conclusion
The results show that pioglitazone can inhibit LPS-stimulated microglia inflammation by blocking p38 MAPK signaling pathway.





Similar content being viewed by others
References
Bernardo A, Ajmone-Cat MA, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome proliferator-activated receptor-c is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J Neurochem. 2005;92:895–903.
Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–60.
Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–7.
Crosby MB, Svenson J, Gilkeson GS, Nowling TK. A novel PPAR response element in the murine iNOS promoter. Mol Immunol. 2005;42:1303–10.
Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-γ agonist 15-deoxy-△12, 14-prostaglandin J2 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–15.
Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor γ Up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Bio Chem. 2007;282:37006–15.
Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome dependent degradation of PPARγ in adipocytes. J Bio Chem. 2002;277:4062–8.
Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15.
Jantaratnotai N, Utaisincharoen P, Piyachaturawat P, Chongthammakun S, Sanvarinda Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006;78:571–7.
Kremlev SG, Palmer C. Interlekin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol. 2005;162:71–80.
Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 Is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86.
Nick JA, Young SK, Brown KK, Avdi NJ, Arndt PG, Suratt BT, Janes MS, Henson PM, Worthen GS. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol. 2000;164:2151–9.
Paintlia AS, Paintlia MK, Singh I, Singh AK. IL-4-induced peroxisome proliferator-activated receptor γ activation inhibits NF-κB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol. 2006;176:4385–98.
Paintlia AS, Paintlia MK, Khan M, Vollmer T, Singh AK, Singh I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. FASEB J. 2005;19:1407–21.
Raviv Z, Kalie E, Seger R. MEK5 and ERK5 are localized in the nuclei of resting as well as stimulated cells, while MEKK2 translocates from the cytosol to the nucleus upon stimulation. J Cell Sci. 2004;117:1773–84.
Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26.
Xing B, Xin T, Hunter RL, Bing G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3 K/Akt. J Neuroinflammation. 2008;5:4.
Xu J, Barger SW, Drew PD. The PPAR-γ agonist 15-deoxy-Δ12,14-prostaglandin J2 attenuatesmicroglial production of IL-12 family cytokines: potential relevance to Alzheimer’s disease, PPAR Res., 2008:Article ID 349185.
Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K. Anti-inflammatory effects of catechols in lipopolysaccharide stimulated microglia cells: inhibition of microglial neurotoxicity. Eur J Pharmacol. 2006;588:106–13.
Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-γ ligands, 15-deoxy-△12, 14-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–37.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.30770488 and No.30870320); Natural Science Foundation of Jiangsu province (No.BK2006547); A special Research Grant(XK 2007 23) for the key laboratory from the Department of Health, Jiangsu Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: L. Li.
Rights and permissions
About this article
Cite this article
Ji, H., Wang, H., Zhang, F. et al. PPARγ agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogen-activated protein kinase signaling pathways. Inflamm. Res. 59, 921–929 (2010). https://doi.org/10.1007/s00011-010-0203-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0203-7