Abstract
Background
CD93 is a cell surface glycoprotein that is required for efficient engulfment of apoptotic cells via an unknown mechanism. Recently, it was demonstrated that CD93 is proteolytically cleaved from the surface of activated human monocytes and neutrophils in response to inflammatory signals in vitro and that a soluble form of CD93 (sCD93) exists in human plasma.
Objective
The objective of this study was to examine the relationship among production of sCD93, inflammation, and engulfment of apoptotic cells.
Methods
Sterile peritonitis was induced in C57BL/6 mice, and lavage fluid was analyzed for presence of sCD93 by ELISA and Western blot. Cellular infiltrate was assessed by flow cytometry and analyzed for its capacity to shed CD93 in vitro. Peritoneal lavage fluid (PLF) was examined for its ability to regulate engulfment of apoptotic cells.
Results
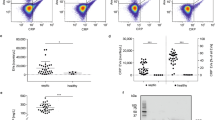
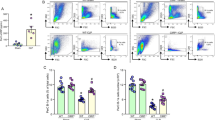
There was an 8.9-fold increase in sCD93 following induction of peritonitis. Macrophages accounted for the majority of infiltrating leukocytes into the peritoneum when sCD93 levels were highest. Inflammatory peritoneal macrophages constitutively shed CD93 in vitro suggesting that this cell type contributes to elevated levels of sCD93 in vivo. Inflammatory PLF from wild-type mice containing elevated sCD93 significantly enhanced engulfment of apoptotic cells in vitro when compared to inflammatory fluid from CD93-deficient mice.
Conclusions
These data demonstrate that inflammation triggers release of sCD93 in vivo, identify the inflammatory macrophage as a source of sCD93, and provide insight into the mechanism by which CD93 contributes to engulfment of apoptotic cells.







Similar content being viewed by others
References
Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004;172:3406–14.
deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–17.
Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94.
Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol. 2007;171:2–8.
Teder P, Vandivier RW, Jiang D, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–8.
Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880–9.
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8.
Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50.
Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–29.
Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol. 2005;175:1239–47.
Jutila MA, Rott L, Berg EL, Butcher EC. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989;143:3318–24.
Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41.
Champagne B, Tremblay P, Cantin A, St PY. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J Immunol. 1998;161:6398–405.
Nagano O, Murakami D, Hartmann D, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902.
Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5.
Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33.
Boehme MW, Werle E, Kommerell B, Raeth U. Serum levels of adhesion molecules and thrombomodulin as indicators of vascular injury in severe Plasmodium falciparum malaria. Clin Investig. 1994;72:598–603.
Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood. 2003;102:1186–95.
Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–85.
Galkina E, Tanousis K, Preece G, et al. l-Selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–35.
Roach T, Slater S, Koval M, et al. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–17.
Lillis AP, Greenlee MC, Mikhailenko I, et al. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 2008;181:364–73.
Kask L, Trouw LA, Dahlback B, Blom AM. The C4b-binding protein-protein S complex inhibits the phagocytosis of apoptotic cells. J Biol Chem. 2004;279:23869–73.
Nauta AJ, Castellano G, Xu W, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173:3044–50.
Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van KC. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–7.
Park M, Tenner AJ. Cell surface expression of C1qRP/CD93 is stabilized by O-glycosylation. J Cell Physiol. 2003;196:512–22.
Guo L, Eisenman JR, Mahimkar RM, et al. A proteomic approach for the identification of cell-surface proteins shed by metalloproteases. Mol Cell Proteomics. 2002;1:30–6.
Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9.
Fonseca MI, Carpenter PM, Park M, Palmarini G, Nelson EL, Tenner AJ. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J Leukoc Biol. 2001;70:793–800.
Dean YD, McGreal EP, Gasque P. Endothelial cells, megakaryoblasts, platelets and alveolar epithelial cells express abundant levels of the mouse AA4 antigen, a C-type lectin-like receptor involved in homing activities and innate immune host defense. Eur J Immunol. 2001;31:1370–81.
Shi CS, Shi GY, Hsiao SM, et al. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;112:3661–70.
Greenlee MC, Sullivan SA, Bohlson SS. CD93 and related family members: their role in innate immunity. Curr Drug Targets. 2008;9:130–8.
Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95.
Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–50.
Chevrier S, Genton C, Kallies A, et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci USA. 2009;106:3895–900.
Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43.
Babic AM. Extracorporeal photopheresis: lighting the way to immunomodulation. Am J Hematol. 2008;83:589–91.
Acknowledgments
The authors thank Jessica Morris, Ana Kozmar, Michael Kuelbs, Jonah Smith, and Mark Wacker for excellent technical assistance. These studies were initiated in the laboratory of Dr. Andrea Tenner at University of California Irvine. CD93−/− mice were provided by Dr. Marina Botto at Imperial College, London. This work was supported by American Heart Association Scientist Development Grant 0630068N to S.S.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: M. Parnham.
Rights and permissions
About this article
Cite this article
Greenlee, M.C., Sullivan, S.A. & Bohlson, S.S. Detection and characterization of soluble CD93 released during inflammation. Inflamm. Res. 58, 909–919 (2009). https://doi.org/10.1007/s00011-009-0064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0064-0