Abstract
The killer immunoglobulin-like receptor (KIR) genes KIR2DL4, KIR3DL2, and KIR3DP1 are present in virtually all humans. KIR2DL4 encodes a receptor present on uterine and decidual natural killer (NK) cells and some peripheral blood NK cells. Its only known ligand is the human leukocyte antigen-G molecule expressed on extravillous trophoblasts, and on tissues in some diseases. KIR3DL2 binds HLA-A*03 and HLA-A*11 as well as HLA-B*27 dimers, and microbial CpG DNA. KIR3DP1 is a pseudogene. During our immunogenetic studies we found two individuals, one from Lower Silesia district in Poland, and another from Western Ukraine, who were reproducibly negative for KIR2DL4 and KIR3DP1 genes, using three different PCR systems. Both individuals displayed very similar genotypes, possessing only KIR3DL3, KIR2DL3, KIR2DP1, KIR2DS1, and probably a rare variant of KIR2DL1. The Pole had also KIR3DL2, which the Ukrainian was apparently lacking. The Lower Silesia has been populated after the Second World War by a remarkable percentage with displaced people from Western Ukraine, which might contribute to genetic similarity of the two individuals described here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Killer cell immunoglobulin-like receptors (KIRs) are present on natural killer (NK) cells and some subpopulations of T lymphocytes. They have either inhibitory (long cytoplasmic tail-receptors KIR2DL and KIR3DL possessing two or three extracellular immunoglobulin-like domains, respectively) or activating (short cytoplasmic tail-receptors KIR2DS or KIR3DS) properties upon binding a ligand. Genes for majority of KIRs are not present in all individuals but only in a fraction of them (haplotypic polymorphism). However, some, so called framework genes, namely KIR3DL3, KIR3DP1, KIR2DL4, and KIR3DL2, are present in virtually all genotypes (Jiang et al. 2012). Among these, KIR2DL4 gene has special properties: it codes for structural features characteristic for both inhibitory and activating KIR; it is expressed in all NK cells whereas other KIRs are rather distributed clonally on some NK cells; and in contrast to other KIRs which function as cell surface receptors, it is localized, in resting NK cells, in endosomes where it may bind its ligand, a soluble HLA-G molecule (Rajagopalan and Long 2012), although this view has been recently challenged (Le Page et al. 2014). KIR2DL4 molecule appears as a cell surface receptor on uterine and decidual NK cells and some (mostly activated) peripheral blood NK cells (Goodridge et al. 2009; Hromadnikova et al. 2013; Yan et al. 2007). KIR3DL2 binds HLA-A*03 and HLA-A*11 as well as HLA-B*27 dimers, and also a microbial CpG DNA (Shaw and Kollnberger 2012; Sivori et al. 2010). KIR3DP1 is a pseudogene (Jiang et al. 2012). Here, we describe two cases of KIR2DL4-negative individuals detected in the Polish and Ukrainian populations. Both individuals present similar deletion of not only KIR2DL4 but also neighboring KIR genes, including KIR3DP1. Interestingly, the Ukrainian individual was also lacking another framework gene, KIR3DL2.
Materials and Methods
Study Subjects
The study was approved by the Local Ethical Committee at the Research and Development Centre, Regional Specialised Hospital, Wrocław, the Bioethics Committee of the Medical University of Poznań, and the Bioethics Committee of the Danylo Halytsky Lviv National Medical University.
No. 89 a 69-year-old Polish male, 82 kg body weight, 171 cm high, body mass index (BMI) of 28.04, with arterial hypertension, morbus ischemicus cordis and aspirin idiosyncrazy but otherwise healthy and without genetic diseases in family.
No. 175 K a 67-year-old Polish male, 90 kg body weight, 177 cm high, BMI of 28.73, with total cholesterol and glucose at upper border of the norm, atherosclerosis, polycystic kidney disease, varices, nosebleed, and osteoporosis.
No. 24 a 22-year-old healthy male from western Ukraine, HIV-, HBV- and HCV-negative blood donor, recruited to a control group for our study on cryptorchidism (Kurpisz et al. 2011).
No. 58 a 19-year-old healthy male from western Ukraine, HIV-, HBV- and HCV-negative blood donor, recruited to a control group for our study on cryptorchidism (Kurpisz et al. 2011).
DNA Isolation
Genomic DNA was isolated from venous blood using QIAamp DNA Blood Mini Kit Qiagen (Qiagen GmbH, Hilden, Germany), according to manufacturer’s recommendations. DNA concentration was measured in NanoDrop 2000 UV–Vis Spectrophotometer (Thermo Scientific, NanoDrop Products, Wilmington, Delaware, USA).
KIR Typing
Three PCR-sequence-specific primers (SSP) systems were used
-
(a)
Olerup SSP KIR Genotyping kit (Olerup GmbH, Vienna, Austria), lot No. 07 N including Taq polymerase. PCR-SSP procedure was performed according to manufacturer’s instruction.
-
(b)
SSP as described by Vilches et al. (2007) that enable to amplify short DNA fragments (96–158 bp), in our modification (Niepieklo-Miniewska et al. 2013)
-
(c)
Multiplex PCR-SSP (four PCR reactions containing primers for 3–4 KIR genes each) as published earlier (Nowak et al. 2011; Sun et al. 2004).
Our KIR typing has been validated three times per year by the International KIR Exchange program organized by the Immunogenetics Center of the University of California at Los Angeles.
Results
Upon genomic KIR typing using commercial Olerup kit (see Materials and Methods) in disease association studies on Polish and Ukrainian patients and respective control groups, we noticed a lack of KIR2DL4 gene in one Polish (No. 89 K) and one Ukrainian (No. 24) control individual (Fig. 1). All other samples from both control and patient groups were positive (Fig. 1). The individual No. 24 apparently was lacking another framework gene, KIR3DL2, whereas another Ukrainian control individual, No. 58, possessed both genes (Fig. 1). To confirm this result, we repeated KIR typing using Olerup kit once more, with the same result (data not shown, and see Table 1). Furthermore, we checked the presence of KIR2DL4 and KIR3DL2 genes in these samples using primers published by Vilches et al. (2007) (Fig. 2). Finally, we typed these samples again with the use of multiplex PCR-SSP described previously (Sun et al. 2004) (Fig. 3). Our individuals No. 89 K and No. 24 were reproducibly KIR2DL4-negative in all these tests, in contrast to other individuals who were all positive (Fig. 1, 2, 3, and results not shown). They also lacked KIR3DP1 which is present whenever KIR2DL4 is present, with extremely rare exceptions (Jiang et al. 2012). They possessed KIR3DL3, 2DL3, 2DP1, and 2DS1, and No. 89 K had also KIR3DL2, absent from No. 24 genotype. Their genotypes contained KIR2DL3 gene, characteristic for the centromeric segment of the KIR region, but another centromeric gene, KIR2DL1, gave inconsistent results: it was detected by multiplex test (Fig. 3; Table 2) but neither by commercial test (Fig. 1; Table 1) nor by PCR-SSP using primers described by Vilches et al. (2007) (Fig. 2).
Results of KIR typing using Olerup KIR Genotyping kit™. Interpretation of all results is shown in Table 1 and described in the text. M: marker of the molecular mass; NC: negative control. Polish individual No. 89 K; Ukrainian individual No. 24; Ukrainian individual No. 58
Results of KIR typing using primers and PCR conditions described by Vilches et al. (2007). a KIR2DL4 typing of samples No. 89 K, No. 24 and two unrelated KIR2DL4-positive individuals. Both No. 89 K (lane 1) and No. 24 (lane 2) were negative (only internal control gave a product), whereas both positive control samples (lanes 3 and 4) gave KIR2DL4 amplicons. b KIR3DL2 typing of the same samples. Only sample No. 24 (lane 2) was negative for framework gene KIR3DL2, whereas both sample No. 89 K (lane 1) and unrelated positive control samples (lanes 3 and 4) gave positive reactions. c KIR2DL1 typing of the same samples. Only control sample shown in lane 3 was positive, whereas all other samples gave no product
Results of KIR typing using multiplex PCR-SSP kit (Sun et al. 2004). Samples No. 89 K (lanes 2, 5, 8 and 11), No. 24 (lanes 3, 6, 9 and 12) and framework gene-positive control sample No. 175 K (lanes 4, 7, 10 and 13) were tested for the presence of particular KIR gene groups as shown, together with results, in Table 2. Please note the absence of KIR2DL4 (G1) in samples No. 89 K and 24, and absence of KIR3DL2 (G3) in sample No. 24. KIR2DL1 (G1) gave clearly positive band in samples No. 89 K and 24 in this test, in contrast to negative results of these samples with both Olerup (Fig. 1) and Vilches et al. (2007) (Fig. 2) primers. Summary of these results is given in Table 2
Discussion
We have detected two KIR3DP1, KIR2DL4-negative individuals, one of which was also negative for the third framework gene, KIR3DL2. Such genotypes are extremely rare in world populations (Djulejic et al. 2010; Ewerton et al. 2007; Gomez-Lozano et al. 2003; Gonzalez-Galarza et al. 2011; Karlsen et al. 2007; Norman et al. 2002; Nowak et al. 2011; Ozturk et al. 2012; Taniguchi and Kawabata 2009; Traherne et al. 2010; Velickovic et al. 2006), and even haplotypes contributing to them, most frequently in heterozygotic configuration, are very rare (Gonzalez-Galarza et al. 2011; Jiang et al. 2012; Pyo et al. 2010; 2013; Traherne et al. 2010). We found in the www.allelefrequencies.net database (Gonzalez-Galarza et al. 2011) only ten KIR2DL4-negative genotypes in addition to two new ones described here (Fig. 4a), three KIR3DL2-negative genotypes plus our Ukrainian (Fig. 4b), and six KIR3DP1-negative genotypes plus two described here (Fig. 4c) out of nearly 13,000 individuals tested worldwide (Gonzalez-Galarza et al. 2011). This speaks in favor of a strong positive selection for all three genes, including KIR3DP1. This pseudogene might be suspected to be preserved simply due to its close proximity to KIR2DL4 which is likely strongly selected for. However, KIR3DP1 is located on centromeric segment of KIR locus, whereas KIR2DL4 is on telomeric segment (Jiang et al. 2012; Pyo et al. 2013) which seems to exclude the close localization as a reason for preservation of KIR3DP1 together with KIR2DL4. Nevertheless, genotypes containing KIR2DL4, but not KIR3DP1, are extremely rare, as only few such genotypes from Cook, Samoa, Tokelau, and Tonga Islands as well as from Turkey have been described so far (Velickovic et al. 2006) (Fig. 4c), and opposite configuration, KIR3DP1 +, KIR2DL4 –, was found in only single individuals from South Turkey, Brazil, and Bulgaria (Fig. 4a). Homozygous KIR3DL2 deletion is also very infrequent, as only three such cases exist in the database (Gonzalez-Galarza et al. 2011) and our Ukrainian No. 24 is the fourth one (Fig. 4b). Haplotypes with this deletion, but not in homozygous configuration, have been identified in some individuals by gene copy number detection (Pyo et al. 2013). The KIR3DL2 molecule plays an important role in both innate and adaptive immunity. It is expressed on NK cells, as well as on subpopulation(s) of T lymphocytes (Bowness et al. 2011; Wong-Baeza et al. 2013). It binds not only HLA-A*03 and HLA-A*11 heterotrimers as well as HLA-B*27 homodimers, but also microbial CpG DNA (Shaw and Kollnberger 2012; Sivori et al. 2010). It has the highest number of alleles, except for KIR3DL1/S1. This is suggestive of a strong selection for polymorphism (Shaw and Kollnberger 2012).
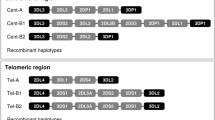
KIR genotypes of published framework KIR-negative individuals. a KIR2DL4-negative individuals. Gray shadowed cells gene present, white diagonally crossed cells gene absent, question mark gene presence dubious (inconsistent results), ND not done. b KIR genotypes of published KIR3DL2-negative individuals. Designations of cells as in Fig. 4a. c KIR genotypes of published KIR3DP1-negative individuals. Designations of cells as in Fig. 4a. References: 1 Gomez-Lozano et al. 2003, 2 Nowak et al. 2011, 3 Gonzalez-Galarza et al. 2011, 4 Norman et al. 2002, 5 Taniguchi and Kawabata 2009, 6 Traherne et al. 2010, 7 Ewerton et al. 2007, 8 Karlsen et al. 2007 9 Ozturk et al. 2012 10 Djulejic et al. 2010, 11 Velickovic et al. 2006
In some very rare American haplotypes, most of the KIR3DL2 gene (intron 3 to beyond exon 9) was deleted, giving a typing pattern identical to our No. 24. Such a haplotype has also been detected in an American of Ukrainian descent, in addition in homozygous configuration (Traherne et al. 2010).
Interestingly, Lower Silesia was populated in remarkable fraction, after the Second World War, by people resettled from Western Ukraine. Therefore, not only No. 24 but also No. 89 K genotype may be derived from there, whence similarity between these genotypes and with haplotypes described in the USA and mentioned above. It has been established by sequencing that all American KIR3DL2 deletion haplotypes contained exons 1–3 from KIR3DL2*007 allele, suggesting a recent common origin. It would be desirable then to check whether our genotypes No. 89 K and No. 24 bear the same allele that may be derived from the same recombination event.
We had a problem with establishing whether KIR2DL1 gene is present in our genotypes No. 89 K and No. 24. The reagents from both Olerup and Vilches et al. (2007) PCR-SSP gave reproducibly negative results, whereas in the multiplex assay both individuals were clearly positive (Figs. 1, 2, 3; Tables 1, 2). It is likely that both our individuals possess genotypes similar to the haplotypes described earlier in Northern American Caucasian population (Pyo et al. 2013; Traherne et al. 2010) which contained a KIR2DL1/KIR2DS1 hybrid resulting from recombination event which deleted all KIR genes located between these two genes. This may explain why some PCRs detected KIR2DL1 in our samples, but other did not.
In conclusion, we found in our Slavic populations (Polish and Ukrainian), two individuals lacking several framework KIR genes, KIR3DP1, and KIR2DL4, and apparently also KIR2DL1. One of them was also negative for KIR3DL2. Both these individuals were relatively healthy in spite of very limited KIR gene repertoire. However, such genotypes are extremely rare in world populations. Therefore, studies on much larger populations would be required to determine the effect(s) of these deletions on the health of such individuals.
References
Bowness P, Ridley A, Shaw J et al (2011) Th17 cells expressing KIR3DL2 + and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol 186:2672–2680
Djulejic E, Petlichkovski A, Trajkov D et al (2010) Distribution of killer cell immunoglobulinlike receptors in the Macedonian population. Hum Immunol 71:281–288
Ewerton PD, Leite Mde M, Magalhaes M et al (2007) Amazonian Amerindians exhibit high variability of KIR profiles. Immunogenetics 59:625–630
Gomez-Lozano N, de Pablo R, Puente S et al (2003) Recognition of HLA-G by the NK cell receptor KIR2DL4 is not essential for human reproduction. Eur J Immunol 33:639–644
Gonzalez-Galarza FF, Christmas S, Middleton D et al (2011) Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res 39(Database issue):D913–D919
Goodridge JP, Lathbury LJ, John E et al (2009) The genotype of the NK receptor, KIR2DL4, influences IFNgamma secretion by decidual natural killer cells. Mol Hum Reprod 15:489–497
Hromadnikova I, Pirkova P, Sedlackova L (2013) Influence of in vitro IL-2 or IL-15 alone or in combination with Hsp-70-derived 14-mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors. Mediators Inflamm 2013:405295
Jiang W, Johnson C, Jayaraman J et al (2012) Copy number variation leads to considerable diversity for B but not A haplotypes of the human KIR genes encoding NK cell receptors. Genome Res 22:1845–1854
Karlsen TH, Boberg KM, Olsson M et al (2007) Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol 46:899–906
Kurpisz M, Nakonechnyy A, Niepieklo-Miniewska W et al (2011) Weak association of anti-sperm antibodies and strong association of familial cryptorchidism/infertility with HLA-DRB1 polymorphisms in prepubertal Ukrainian boys. Reprod Biol Endocrinol 9:129
Le Page MEL, Goodridge JP, John E et al (2014) Killer Ig-like receptor 2DL4 does not mediatte NK cell IFG-γ responses to soluble HLA-G preparations. J Immunol 192:732–740
Niepieklo-Miniewska W, Majorczyk E, Matusiak L et al (2013) Protective effect of the KIR2DS1 gene in atopic dermatitis. Gene 527:594–600
Norman PJ, Carrington CV, Byng M et al (2002) Natural killer cell immunoglobulin-like receptor (KIR) locus profiles in African and South Asian populations. Genes Immun 3:86–95
Nowak I, Majorczyk E, Ploski R et al (2011) Lack of KIR2DL4 gene in a fertile Caucasian woman. Tissue Antigens 78:115–119
Ozturk OG, Polat G, Atik U (2012) Diversity of killer cell immunoglobulin-like receptor genes in Southern Turkey. Mol Biol Rep 39:1989–1995
Pyo CW, Guethlein LA, Vu Q et al (2010) Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS ONE 5:e15115
Pyo CW, Wang R, Vu Q et al (2013) Recombinant structures expand and contract inter and intragenic diversification at the KIR locus. BMC Genom 14:89
Rajagopalan S, Long EO (2012) KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol 3:258
Shaw J, Kollnberger S (2012) New perspectives on the ligands and function of the killer cell immunoglobulin-like receptor KIR3DL2 in health and disease. Front Immunol 3:339
Sivori S, Falco M, Carlomagno S et al (2010) A novel KIR-associated function: evidence that CpG DNA uptake and shuttling to early endosomes is mediated by KIR3DL2. Blood 116:1637–1647
Sun JY, Gaidulis L, Miller MM et al (2004) Development of a multiplex PCR-SSP method for Killer-cell immunoglobulin-like receptor genotyping. Tissue Antigens 64:462–468
Taniguchi M, Kawabata M (2009) KIR3DL1/S1 genotypes and KIR2DS4 allelic variants in the AB KIR genotypes are associated with Plasmodium-positive individuals in malaria infection. Immunogenetics 61:717–730
Traherne JA, Martin M, Ward R et al (2010) Mechanisms of copy number variation and hybrid gene formation in the KIR immune gene complex. Hum Mol Genet 19:737–751
Velickovic M, Velickovic Z, Dunckley H (2006) Diversity of killer cell immunoglobulin-like receptor genes in Pacific Islands populations. Immunogenetics 58:523–532
Vilches C, Castano J, Gomez-Lozano N et al (2007) Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens 70:415–422
Wong-Baeza I, Ridley A, Shaw J et al (2013) KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J Immunol 190:3216–3224
Yan WH, Lin A, Chen BG et al (2007) Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am J Reprod Immunol 57:233–242
Acknowledgments
The research described in this paper was supported by two projects: WROVASC––“Integrated Centre of Cardiovascular Medicine” co-founded by the European Fund of Regional Development and national budget (the Innovative Economy Operational Program 2007–2013 1-1), and a grant from the Polish Ministry of Science and Higher Education No. NN407193439.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Niepiekło-Miniewska, W., Żuk, N., Dubis, J. et al. Two New Cases of KIR3DP1, KIR2DL4-Negative Genotypes, One of which is also Lacking KIR3DL2 . Arch. Immunol. Ther. Exp. 62, 423–429 (2014). https://doi.org/10.1007/s00005-014-0299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-014-0299-5