Abstract
Peritoneal dialysis is one of the main modality of treatment in end-stage kidney diseases (ESKD) in children. In our previous work in chronic kidney disease patients, in pre-dialyzed period and on hemodialysis, the neutrophils were highly activated. The aim of this study was to assess an inflammatory condition and neutrophil activation in ESKD patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Thirteen CAPD patients without infection, both sexes, aged 2.5–24 years, and group of healthy subjects (C) were studied. For comparative purposes the conservatively treated (CT) group of ESKD patients was included. Neutrophil elastase in complex with α1-proteinase inhibitor (NE-α1PI; ELISA), α1-proteinase inhibitor (α1PI; radial immunodiffusion) and interleukin-8 (IL-8; ELISA) were measured in the blood samples from CAPD, CT, and C group and in the peritoneal dialysate fluid (PDF) samples of patients on CAPD. A significantly increased plasma NE-α1PI levels (median 176.5 μg/L, range 85.2–373.2 μg/L; p < 0.00005), serum IL-8 (median 18.6 pg/mL, range 15.73–35.28 pg/mL; p < 0.05), and slightly decreased serum α1PI (median 1,540 mg/L, range 1,270–1,955; p ≤ 0.05) compared to the control groups were found. There were no significant differences of analyzed parameters between CAPD and CT patients. The concentration ratio of NE-α1PI, α1PI and IL-8 in blood/PDF was 29.97, 8.24, and 4.48, respectively. There were significantly positive correlations between serum and PDF concentration of α1PI and IL-8 (r = 0.613, p < 0.05; r = 0.59; p < 0.005, respectively). The results of our study demonstrate that neutrophils are highly activated in non-infected CAPD patients. The pivotal marker of this activation is NE-α1PI. It may contribute to chronic inflammation and tissues injury.
Similar content being viewed by others
Introduction
Uremia and long-term continuous ambulatory peritoneal dialysis (CAPD) may result in neutrophil disorders regarding their quantity, morphological or functional changes (Santamaria et al. 2009; Wasik et al. 1997). As a result, this leads to higher risk of developing inflammatory conditions, such as peritonitis and catheter-related infection (Stefaniak et al. 2002) as well as non-inflammatory complications including cardiovascular disease, fibrosis, impaired peritoneal ultrafiltration and an increase in mortality (Litwin et al. 2001; Yamamoto et al. 2010). Inflammation usually is associated with an overstimulation of neutrophils and release of their proteolytic enzymes, accompanied by the imbalance between cellular proteases and their inhibitors, and the activation of pro-inflammatory cytokines as well (Augustyniak et al. 2006; Korkmaz et al. 2005a; Lin and Huang 1994; Polańska et al. 2007). The hallmarks of neutrophilic inflammation are neutrophil elastase, α1-proteinase inhibitor (α1PI) and interleukin-8 (IL-8).
Free human neutrophilic elastase is regarded as one of the most potent proteolytic enzymes. It plays an important physiological function, being able to degrade foreign phagocytized particles both intracellularly (Shapiro 2002) and extracellularly (Papayannopoulos et al. 2010) and facilitate cell migration through vascular walls (Kaynar et al. 2008). One of the major and natural inhibitors of elastase is α1PI (Korkmaz et al. 2005a), which protects the surrounding tissues from enzyme-mediated destruction. Neutrophil elastase, both in the free form and in enzymatically, inactive complex with α1PI, may be a good indicator of the neutrophil activity (Polańska et al. 2004) as well as the degree of inflammatory reactions in acute and chronic diseases, including kidney disorders requiring dialysis (Donovan et al. 1993; Polańska et al. 2006, 2010; Shutov et al. 1999). IL-8 is the major chemotactic factor for neutrophils which also participates in their activation (Baggiolini and Clark-Lewis 1992) and degranulation (Segura et al. 1998). It also plays a significant role in the pathogenesis of inflammatory diseases (Baggiolini and Clark-Lewis 1992), cardiovascular diseases (Apostolakis et al. 2009) and fibrosis (Masunaga et al. 2003).
Although neutrophil elastase, α1PI and IL-8 play an important role in modulation of inflammation, the number of data referring to their significance in pediatrics patients with chronic kidney disease (CKD) is highly limited. Despite excessive data concerning the role of aforementioned mediators in kidney diseases analyzed separately, little is known about interrelations between them in pediatric patients in reference to renal replacement therapies. The goal of our study was to evaluate the blood and peritoneal dialysate fluid (PDF) concentrations of NE-α1PI, α1PI, and IL-8 in non-infected children and young adults with CKD on CAPD searching for differences between those parameters.
Materials and Methods
Subjects
The study covered 13 children and young adults with chronic and end-stage kidney disease (ESKD) on CAPD patients, aged 2.5–24 years (12.1 ± 6.8 years, mean ± SD), 7 females (54 %), and 6 males (46 %). The patients were dialyzed from 0.5 to 10 years (3.8 ± 3.6 years, mean ± SD) in the Department of Pediatric Nephrology in Wroclaw, Poland. All patients used conventional, low-glucose peritoneal dialysis solutions. Clinical causes which led to a CAPD therapy are shown in Table 1. All patients were clinically stable. In the time of investigation there were no clinical and conventional laboratory (C-reactive protein, erythrocyte sedimentation rate, total leukocytes count) signs of infection. None of the patients enrolled in the study had immunologic abnormalities. The patients with autoimmune disease had complete clinical and biochemical remission. Additionally, for comparative purposes the previously published conservatively treated (CT) group was included (Polańska et al. 2010). The CT group consisted of 13 patients, 6 females (46 %) and 7 males (54 %), aged 4–17 years (mean 12 ± 4.5 years), The causes of CKD in the CT group were: chronic glomerular nephritis (n = 4), hydronephrosis (n = 1), polycystic kidney disease (n = 4), kidney hypoplasia (n = 1), chronic interstitial nephritis (n = 1), and posterior urethral valves (n = 2). The control (C) group consisted of healthy subjects, both sexes, without chronic or recurrent diseases in anamnesis. Their results were regarded as normal values for NE-α1PI (group C1, n = 40, age range 1–16, mean 7 ± 4 years), for α1PI (group C2, n = 29, age range 1–24, mean 10 ± 7 years), and for IL-8 (group C3, n = 38, age range 1–24, mean 13.5 ± 7.5 years). None of the patients and controls received drugs having potential anti-inflammatory properties.
Material and Sampling Procedures
The material for investigation included peripheral venous blood which was obtained along with the blood drawn for routine laboratory tests from CAPD patients (B-CAPD), CT and healthy subjects, and included PDF from the abdominal cavity drawn simultaneously from CAPD patients (PDF-CAPD). Samples from PDF were obtained after 4 h dwell time. The blood samples, EDTA-treated blood samples and PDF were centrifuged (3,000 rpm, 10 min) within 2 h after collection. The serum, plasma and PDF were immediately divided into aliquots and stored at −80 °C until assayed.
This study was approved by the Research Ethics Committee of the Medical University of Wroclaw. The children were enrolled for the study with parental agreement. Informed consent was obtained from each patient’s parent and adult patients.
Methods
Neutrophil elastase was determined by ELISA method in plasma and in undiluted PDF as a complex with its natural inhibitor, α1-proteinase using reagents manufactured by Merck (Darmstadt, Germany). The serum and undiluted PDF concentration of α1PI and IL-8/NAP-1 were investigated by radial immunodiffusion method using the Binding Site kit (Birmingham, UK), and ELISA kit of Bender MedSystems (Vienna, Austria), respectively. The analyses were performed according to the manufacturers’ recommendations. The limits of detection for NE-α1PI and IL-8/NAP-1 were <1.98 μg/L and <11 pg/mL, respectively. The coefficient of variation of α1PI repeat measurements was <5 %.
Statistical Analysis
Statistical analysis was performed using the nonparametric the Mann–Whitney U test for independent variables. Spearman’s rank correlation coefficient was used to investigate any relationship between the parameters. The level of statistical significance was assumed to be p < 0.05. The analyses and illustrations were performed using StatSoft software (Statistica 8).
Results
In all the analyzed samples of blood and PDFs investigated markers were detectable (Tables 1, 2). Significantly higher plasma NE-α1PI (p < 0.00005) and serum IL-8 (p < 0.05) levels were observed in CAPD patients in comparison to healthy subjects. In 4/13 cases (31 %) plasma NE-α1PI levels were within the normal range (60.78 ± 54.20 μg/L; mean ± 2SD) and in 5/13 (38 %) cases levels were above 200 μg/L. The median values of α1PI was slightly, but significantly (p ≤ 0.05) below the accepted norms. In the majority of samples [10/12 (83 %)] concentrations of α1PI were within the normal limits (1,862.01 ± 825.8 mg/L; mean ± 2SD). Particularly low concentrations of α1PI we found in the blood of two patients who had simultaneously elevated levels of NE-α1PI [first patient with vesicoureteral reflux (α1PI 169 mg/L, NE-α1PI 537.5 μg/L) and second with congenital nephrotic syndrome (α1PI 932 mg/L, NE-α1PI 422.3 μg/L)]. The analysis of α1PI in CAPD group depleted of at least one mentioned serum samples versus controls abolishes the indicated above significance. Serum IL-8 was above the reference range (17.41 ± 26.18 pg/mL, mean ± 2SD) in 3/13 (23 %) patients. One of them was the same person who had simultaneously elevated concentration of NE-α1PI and α1PI (with vesicoureteral reflux, IL-8 124.15 pg/mL, NE-α1PI 537.5 μg/L, α1PI 169 mg/L). There were no significant differences between B-CAPD and CT patients in reference to all three measured indicators (Table 2). The differences between CT patients and controls have been published previously (Polańska et al. 2010).
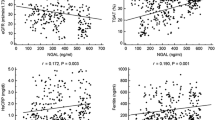
The B-CAPD levels of NE-α1PI were 30-fold higher, α1PI, eightfold higher whereas IL-8 only 4.5-fold higher comparing to their counterparts in PDF-CAPD. All tested inflammatory markers were in much lower concentrations in the PDF-CAPD compared to their levels accepted as the normative values in peripheral blood (NE-α1PI and α1PI about 10 times, IL-8 3.5 times lower). There were significant positive correlation between serum and PDF concentration of α1PI (r = 0.613, p < 0.05) and IL-8 (r = 0.59, p < 0.005), see Fig. 1. There were no correlations between the blood concentration of NE-α1PI, α1PI and IL-8 and between plasma and PDF concentrations of NE-α1PI. No statistically significant differences in concentrations of NE-α1PI, α1PI and IL-8 between the blood, PDF and duration of CAPD therapy were found.
Considering the potential influence of congenital anomalies of kidney and urinary tract (CAKUT) in CAPD patients on tested parameters, we did not find any significant differences between CAKUT group (Table 1: no. 1, 2, 4, 5, 10, 13) and that with other underlying diseases.
Discussion
In long-therapy of CAPD patients with ESKD, the competent systemic and local, peritoneal immune defense mechanisms are very important. The results of our previous data clearly showed the high activity of neutrophils in young ESKD patients on hemodialysotherapy as well as in conservatively treated patients. It has been confirmed by considerable increase in circulatory level of NE-α1PI (Polańska et al. 2010). The results of our present study indicate that also in CAPD patients we found elevated levels of NE-α1PI in plasma. At the same time the concentration of NE-α1PI in the PDF were approximately tenfold lower in comparison to normal plasma levels, suggesting higher activity of neutrophils in peripheral blood than in the peritoneal cavity.
Due to the variety of relationships and interactions between the cells and their metabolites it is difficult to clearly identify the causes and the clinical consequences of increase activation of neutrophils in blood. The results of our study revealed that CAPD patients have a tendency to possess higher values of NE-α1PI and IL-8 than CT ones as compared to normal. We can only speculate that this is presumably in part a result of the body response to stimulating action of both the not fully removed circulated uremic toxins, invasiveness of the peritoneal dialysis procedure, inbiocompatibility dialysis solutions and IL-8 activities. Furthermore, it may be also connected with the property of neutrophil elastase in complex with α1PI to serve as a neutrophil chemoattractant (Banda et al. 1988). We can also assume that in the peritoneal cavity of our patients there was no acute inflammation which can over-stimulate neutrophils. The concentration of NE-α1PI and free elastase activity determined in the peritoneal cavity has already been studied in patients without peritonitis, showing the reduction of both form of enzyme (Donovan et al. 1993). Conversely, in peritonitis a common complication of CAPD in children, elevated levels of elastase both in plasma and dialysate fluid were observed (Shutov et al. 1999).
The consequences of observed NE-α1PI increase in pediatric patients for whom peritoneal dialysis is often a method of choice (especially in smaller children) may be diverse. Firstly, as the dynamic changes in the concentration of free neutrophil elastase or NE-α1PI in body fluids are very sensitive, its excessive amount may have significant importance for the preservation of homeostasis. Secondly, when elastase is released from overactivated neutrophils or after their apoptosis, unbounded neutrophil elastase can destroy extracellular matrix, basement membranes (Chua and Laurent 2006), and promote microvascular injury (Carden et al. 1998). This proteolytic feature of enzyme may prevent from fibrosis development, one of the most serious side effects of the long-term peritoneal dialysis (Yamamoto et al. 2010). On the other hand, however, neutrophil elastase paradoxically participates in excessive extracellular matrix deposition that leads to pulmonary fibrosis (Yamanouchi et al. 1998). Furthermore, neutrophil elastase inactivates complement components C3 and C5a, immunoglobulins, protease inhibitors, clotting factors and cell adhesion molecules that have a direct impact on the course of the inflammatory reactions (Lee and Downey 2001). All aforementioned autoaggressive properties of neutrophil elastase may contribute to the increase of the pathological remodeling of tissues in CKD patients. Thus, even a small amount of free neutrophil elastase present in the peritoneal cavity of our patients may support microinflammation of peritoneal membrane and ultrafiltration failure (Andreoli et al. 1994; Bos et al. 1991; Donovan et al. 1993). The reduced quantity/activity of neutrophil elastase may result also in neutrophil-related endothelial cell injury and increase vasculature permeability (Kaynar et al. 2008).
Neutrophil elastase activity is fully controlled by α1PI and other inhibitors as long as they are present in excess. It is worth to add that about 12 % of neutrophil elastase content in primary neutrophil granules is mobilized to the cell membrane where neutrophil elastase is resistant to inhibitors and remains catalytically active (Korkmaz et al. 2005b; Owen et al. 1995). The results of our study have shown that elevated concentrations of NE-α1PI were not accompanied by the α1PI increase in peripheral blood in both CKD groups. We suspect that this may be only an apparent shortage as a result of the rapid consumption of inhibitor molecules by excessively released neutrophil elastase and subsequent elimination of NE-α1PI complexes (Korkmaz et al. 2005a). Because α1PI is considered as a positive marker of acute inflammatory reaction, its low quantity probably reflects the lack of such a response in our patients. Another explanation may be the proteolytic degradation of α1PI by myeloperoxidase (Honda et al. 2009) or neutrophil elastase (Cantin et al. 1989), indirectly confirmed by the lack of significant correlations between NE-α1PI and α1PI both in the blood and PDF CAPD samples. Finally, α1PI inactivation may be the result of excessive oxidative stress which occurs in patients on dialysis (Zwolińska et al. 2009) followed by the release of free reactive oxygen species (Carp and Janoff 1980).
Identified relatively low concentrations of IL-8 in the serum of CAPD patients, although significantly higher when compared to healthy control (p = 0.036) indicate rather a chronic inflammation or microinflammation than the acute condition. This can be however a real risk for cardiovascular complications (Apostolakis et al. 2009). It is worth noting that in our patients, without clinical manifestations of peritonitis, it was the smallest differences in the concentrations of IL-8 between the serum and the PDF in comparison to NE-α1PI and α1PI. This may be partly the result of the easier compensation of pro-inflammatory cytokines by anti-inflammatory cytokines observed in children (Pereira 1995) or suppressive action of free radicals on the production of IL-8 (DeForge et al. 1992). In accordance with other data we speculate therefore that the source of IL-8 found in the PDF samples may be rather cytokine-activated peritoneal macrophages or fibroblasts (Lin and Huang 1994; Witowski et al. 2001) than neutrophils.
The lack of significant correlation found between IL-8 and NE-α1PI in the blood and PDF can provide that neutrophil degranulation followed by the release of elastase was also influenced by other factors, such as (not determined in this work) the components of complement, leukotrienes, and tumor necrosis factor α. Positive correlation between serum and PDF IL-8 and α1PI concentration, found in investigated samples, may be partly the consequence of the chronic or microinflammation presence in long-term dialysed uraemic patients, even without clinical signs. As demonstrated by Bergin et al. (2010) α1PI may play also an anti-inflammatory role by inhibiting neutrophil chemotaxis. It forms a complex with IL-8, and controls the binding of IL-8 with CXCR1 (neutrophil chemokine receptor). The interaction between neutrophil elastase, inhibitor α1-proteinase and IL-8 contributes to the perpetuating circle that facilitates the recruitment of inflammatory cells and amplifies the neutrophil-associated tissue destruction.
There are some limitations to the present study, thus all conclusions must be assessed carefully. First, issue is the low number of pediatric patients, reflecting the general difficulty in performing clinical studies in this population. Secondly, few of our patients have underlying inflammatory diseases which might affect the evaluated parameters. Thirdly, we did not evaluate the functional activities of the study parameters or other multidirectional cellular and molecular interactions influencing them. We did not perform also laboratory data excluding the primary α1PI deficiency. Finally, because we performed a clinical study, our conclusions are based rather on associations and cannot prove causality.
In conclusion, the results of our study due to the fact of increased concentration of NE-α1PI in peripheral blood and their presence in PDF demonstrate that neutrophils are highly active in non-infected CAPD patients. The investigation of the innate immunity components might show new indices on pathogenesis, identify novel therapeutic targets for treatment, and prevent from many complications improving the life quality of these patients.
Abbreviations
- CAKUT:
-
Congenital anomalies of kidney and urinary tract
- CAPD:
-
Continuous ambulatory peritoneal dialysis
- CT:
-
Conservative treatment
- CKD:
-
Chronic kidney disease
- ESKD:
-
End-stage kidney disease
- PDF:
-
Peritoneal dialysate fluid
- B-CAPD:
-
Blood-CAPD
- α1PIα1 :
-
α1-Proteinase inhibitor
- NE-α1PI:
-
Neutrophil elastase complexed with α1-proteinase inhibitor
- IL-8:
-
Interleukin-8
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Andreoli SP, Mallett C, Williams K et al (1994) Mechanisms of polymorphonuclear leukocyte mediated peritoneal mesothelial cell injury. Kidney Int 46:1100–1109
Apostolakis S, Vogiatzi K, Amanatidou V et al (2009) Interleukin 8 and cardiovascular disease. Cardiovasc Res 84:353–360
Augustyniak D, Majkowska-Skrobek G, Basiewicz-Worsztynowicz B et al (2006) The role of IL-6/sIL-6R complex and its natural inhibitor sgp130 in modulation of inflammatory process. Postepy Biochem 52:194–203
Baggiolini M, Clark-Lewis I (1992) Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 307:97–101
Banda MJ, Rice AG, Griffin GL et al (1988) The inhibitory complex of human alpha1-proteinase inhibitor and human leukocyte elastase is a neutrophil chemoattractant. J Exp Med 167:1608–1615
Bergin DA, Reeves EP, Meleady P et al (2010) α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest 120:4236–4250
Bos HJ, Struijk DG, Tuk CW et al (1991) Peritoneal dialysis induces a local sterile inflammatory state and the mesothelial cells in the affluent are related to the bacterial peritonitis incidence. Nephron 59:508–509
Cantin A, Bilodeay G, Begin R (1989) Granulocyte elastase-mediated proteolysis of α1-antitrypsin in cystic fibrosis bronchopulmonary secretions. Pediatr Pulmonol 7:12–17
Carden D, Xiao F, Moak C et al (1998) Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol 275(2 Pt 2):H385–H392
Carp H, Janoff A (1980) Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest 66:987–995
Chua F, Laurent GJ (2006) Neutrophil elastase: mediator of extracellular matrix destruction and accumulation. Proc Am Thorac Soc 3:424–427
DeForge LE, Fantone JC, Kenney JS et al (1992) Oxygen radical scavengers selectively inhibit interleukin-8 production in human whole blood. J Clin Invest 90:2123–2129
Donovan KL, Pacholok S, Humes JL et al (1993) Intra-peritoneal free elastase in CAPD peritonitis. Kidney Int 44:87–90
Honda H, Ueda M, Kojima S et al (2009) Assessment of myeloperoxidase and oxidative α1-antitrypsin in patients on hemodialysis. Clin J Am Soc Nephrol 4:142–151
Kaynar AM, Houghton AM, Lum EH et al (2008) Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol 39:53–60
Korkmaz B, Poutrain P, Hazouard E et al (2005a) Competition between elastase and related proteases from human neutrophil for binding to α1-protease inhibitor. Am J Respir Cell Mol Biol 32:553–559
Korkmaz B, Attucci S, Jourdan ML et al (2005b) Inhibition of neutrophil elastase by α1-protease inhibitor at the surface of human polymorphonuclear neutrophils. J Immunol 175:3329–3338
Lee W, Downey G (2001) Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med 164:896–904
Lin CY, Huang TP (1994) Gene expression and release of interleukin-8 by peritoneal macrophages and polymorphonuclear leukocytes during peritonitis in uremic patients on continuous ambulatory peritoneal dialysis. Nephron 68:437–441
Litwin M, Grenda R, Prokurat S et al (2001) Patient survival and causes of death on hemodialysis and peritoneal dialysis-single-center study. Pediatr Nephrol 16:996–1001
Masunaga Y, Muto S, Asakura S et al (2003) Ascites from patients with encapsulating peritoneal sclerosis augments NIH/3T3 fibroblast proliferation. Ther Apher Dial 7:486–493
Owen CA, Campbell MA, Sannes PL et al (1995) Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol 131:775–789
Papayannopoulos V, Metzler KD, Hakkim A et al (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 3:677–691
Pereira BJ (1995) Balance between pro-inflammatory cytokines and their specific inhibitors in patients on dialysis. Nephrol Dial Transplant 10(suppl 7):27–32
Polańska B, Sidor D, Leszczyk-Kapusta I et al (2004) Neutrophil elastase and interleukin-8 as inflammatory mediators in mechanically ventilated children. Med Sci Monit 10:CR463–CR468
Polańska B, Niemczuk M, Augustyniak D et al (2006) Plasma neutrophil elastase in children with recurrent aphthous stomatitis. Centr Eur J Immunol 31:15–17
Polańska B, Makulska I, Augustyniak D et al (2007) Serum levels of MMP-9 in children and young adults with chronic kidney disease treated conservatively and undergoing hemodialysis. Centr Eur J Immunol 32:66–71
Polańska B, Augustyniak D, Makulska I et al (2010) Elastase, α1-proteinase inhibitor, and interleukin-8 in pre-dialyzed and hemodialyzed patients with chronic kidney disease. Pediatr Int 52:735–743
Santamaria B, Ucero AC, Benito-Martin A et al (2009) Taming apoptosis in peritoneal dialysis. Perit Dial Int 29(Suppl 2):S45–S48
Segura RM, Alegre J, Varela E (1998) Interleukin-8 and markers of neutrophil degranulation in pleural effusions. Am J Respir Crit Care Med 157(5 Pt 1):1565–1572
Shapiro SD (2002) Neutrophil elastase: path clearer, pathogen killer, or just pathologic? Am J Respir Cell Mol Biol 26:266–268
Shutov EV, Grabskaya ES, Ermolenko VM et al (1999) Elastase activity and alpa-1proteinase inhibitor (PI) in serum and dialysate of CAPD patients. Nephrol Dial Transplant 14(9):A234
Stefaniak E, Warzywoda A, Błaszczyński M et al (2002) Clinical investigations of children with chronic renal failure and peritonitis treated with peritoneal dialysis. Pol Merkur Lekarski 12:276–278
Wasik M, Blaim M, Kolewska D et al (1997) Changes in the phagocytic cells in children treated with continuous ambulatory peritoneal dialysis. Arch Immunol Ther Exp 45:189–194
Witowski J, Thiel A, Dechend R et al (2001) Synthesis of C-X-C and C–C chemokines by human peritoneal fibroblasts: induction by macrophage-derived cytokines. Am J Pathol 158:1441–1450
Yamamoto T, Nagasue K, Okuno S et al (2010) The role of peritoneal lavage and the prognostic significance of mesothelial cell area in preventing encapsulating peritoneal sclerosis. Perit Dial Int 30:343–352
Yamanouchi H, Fujita J, Hojo S et al (1998) Neutrophil elastase: alpha-1-proteinase inhibitor complex in serum and bronchoalveolar lavage fluid in patients with pulmonary fibrosis. Eur Respir J 11:120–125
Zwolińska D, Grzeszczak W, Szczepańska M et al (2009) Oxidative stress in children on peritoneal dialysis. Perit Dial Int 29:171–177
Acknowledgments
Our study was reviewed and approved by the Research Ethics Committee at the Wroclaw Medical University. The study was carried out and financed within the framework of the University Research Project no. 601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Polańska, B., Augustyniak, D., Makulska, I. et al. Elastase, α1-Proteinase Inhibitor, and Interleukin-8 in Children and Young Adults with End-Stage Kidney Disease Undergoing Continuous Ambulatory Peritoneal Dialysis. Arch. Immunol. Ther. Exp. 62, 239–245 (2014). https://doi.org/10.1007/s00005-013-0265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-013-0265-7