Abstract
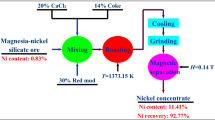
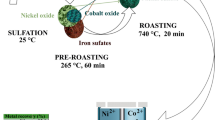
The dissolution behavior of lateritic nickel ore containing 1.2% Ni, 40.6% Fe, 9.5% SiO2 and 9.59% MgO by sulfuric acid was investigated. The ore was characterized adequately by different techniques such as chemical analysis, scanning electron microscope (SEM), X-ray diffraction (XRD), field emission scanning electron microscope (FESEM), thermal analysis and Fourier transform infrared (FTIR) spectroscopic measurements. The characterization results did not reveal any distinct mineral phase for nickel; it was associated with mineral phases like goethite, hematite, chromite and serpentine in various proportions. The beneficiation studies by hydrocyclone, magnetic separation and flotation did not enrich the nickel value. Reduction roasting followed by magnetic separation indicated only 2%Ni with 28.9% yield. Consequently, the recovery of nickel values from the lateritic ore by acid leaching under different conditions was studied. The kinetics of leaching obey the first order rate equations. The recovery of nickel was found to be influenced by temperature. It was observed that at an acid concentration of 2M, leaching temperature 363 K, time 240 min and solid-to-liquid ratio of 1:10, it is possible to leach out more than 95% Ni.
Similar content being viewed by others
References
Al-Mansi, N.M., and Abdel, M.N., 2002, “Recovery of nickel oxide from spent catalyst,” Waste Management, Vol. 22, pp. 85–90.
Ananda Rao, K., Sreenivas, T., Natarajan, R., and Krishna Rao, N., 1995, “Pre concentration of nickel values from lateritic chromite ore overburden, Sukinda, Orissa, India,” Mineral Processing & Extractive Metallurgy Review, Vol. 15, No. (1–4), pp. 37–45.
Baba, A.A., Adekola, A.F., and Bale, R.B., 2009, “Development of a combined pyro- and hydro-metallurgical route to treat spent zinc-carbon batteries,” Journal of Hazardous Materials, Vol. 171, pp. 838–844.
Burger, P.A., 1996, Origins and Characteristics of Lateritic Nickel Deposits, Nickel 96: Mineral to Market, eds. E.J. Grimsey and I. Neuss, Australian Institute of Mining and Metallurgy, 179 pp.
Buyukakinci, E., and Topkaya, Y.A., 2009, “Extraction of nickel from lateritic ores at atmospheric pressure with agitation leaching,” Hydrometallurgy, Vol.9, pp. 33–38.
Caron, M.H., 1950, “Fundamental and practical factors in ammonia leaching of nickel and cobalt ores,” Journal of Metals, Vol. 188, pp. 67–90.
Dalvi, A.D., Bacon, W.G., and Osborne, R.C., 2004, “The past and the future of nickel laterites,” Proceedings of the PDAC International Conference Trade Show and Investors Exchange, Toronto, Canada, Prospectors and Developers Association of Canada, pp. 27.
Das, B., and Reddy, P.S.R., 1990, “Pre concentration of nickel in chromite overburden by hydrocyclone,” Indian Mining and Engineering Journal, Vol. 3, pp. 30–34.
Georgiou, D., and Papangelakis, V.G., 1998, “Sulfuric acid pressure leaching of a limonitic laterite chemistry and kinetics,” Hydrometallurgy, Vol. 49, pp. 23–46.
Habashi, F., 1969, Principles of Extractive Metallurgy, Vol. 1 Gordon & Breach, New York, 11 pp.
Ismail, G., Abdullah, O., and Ayse, U., 2011, “Dissolution behaviour of a Turkish lateritic nickel ore,” Minerals Engineering, Vol. 24, No.7, pp. 603–609.
Iwasaki, I., Takahashi, Y., and Kahata, H., 1966, “Extraction of nickel from iron laterites and oxidized nickel ores by a segregation process,” AIME Transactions, Vol. 235, pp. 308–320.
Kanungo, S.B., 1987, “Recovery of nickel and cobalt from lateritic ores of Orissa, India by selective sulfation roasting,” Transactions of the Institutions of Mining and Metallurgy Section C, Vol. 96, pp. C13–C20.
Kawatra, S.K., and Natarajan K.K., 2001, “Microbial aspect of mineral benefciation, metal extraction and environmental control,” Mineral Biotechnology, 37pp.
Langer, K., and Letteno, D., 1980, “Identifcation of a low-energy OH-valence vibration in zoisite,” American Mineralogist, Vol. 65, pp. 779–783.
Liu, K., Chen, Q., and Hu, H., 2009, “Comparative leaching of minerals by sulfuric acid in a Chinese ferruginous nickel laterite ore,” Hydrometallurgy, Vol. 98, pp. 281–286.
McDonald, R.G., and Whittington, B.I., 2008, “Atmospheric acid leaching of nickel laterites review: Part I. Sulfuric acid technologies,” Hydrometallurgy, Vol. 91, pp. 35–55.
Mohanty, J.K., Rao, D.S., Das, B., and Rao, R.B., 2000, “Mineralogy and pre-concentration of chromite over burden of the Sukinda ultramafc belt, Orissa, India,” CIM Bulletin, Vol. 93, No. 1038, pp. 37–43.
Motlagh, M.M.K., Youzbashi, A.A., and Sabaghzadeh, L., 2011, “Synthesis and characterization of nickel hydroxide/oxide nano particles by the complexation-precipitation method,” International Journal of Physical Sciences, Vol. 6, No. 6, pp. 1471–1476.
Norgate, T., and Jahanshahi, S., 2011, “Assessing the energy and green house gas foot prints of nickel laterite processing,” Minerals Engineering, Vol. 7, No. 24, pp. 698–707.
Perrier, N., Gilkes, R.J., and Colin, F., 2006, “Heating iron rich soils increases the dissolution rate of metals,” Clays and Clay Minerals, Vol. 54, pp. 165–175.
Prasad, P.S.R., Shiva Prasad, K., Krishna Chaitanya, V., Babu, E.V.S.S.K., Sreedhar, B., and Ramana Murthy, S., 2006, “In situ FTIR study on the dehydration of natural goethite,” Journal of Asian Earth Sciences, Vol. 27, pp. 503–511.
Rizov, B., 2012, “Phase transformations from goethite to hematite and thermal decomposition in various nickeliferous laterite ores,” Journal of the University of Chemical Technology and Metallurgy, Vol. 47, No. 2, pp. 207–210.
Suri, A.K., and Gupta C.K., 1995, “Studies on processing of nickeliferrous sources in India,” Mineral Processing & Extractive Metallurgy Review, Vol. 15, No. 1–4, pp. 31–36.
Stopic, S., Friedrich, B., and Fuchs, R., 2003, “Sulfuric acid leaching of the Serbian nickel lateritic ore,” Erzmetall, Vol. 56, pp. 204–209.
Whittington, B.I., and Muir, D., 2000, “Pressure acid leaching of nickel laterites: a review,” Mineral Processing & Extractive Metallurgy, Vol. 21, No. 6, pp. 527–600.
Zhai, X., Fu, Y., Zhang, X., Ma, L., and Xie, F., 2009, “Intensifcation of sulfation and pressure acid leaching of nickel laterite by microwave radiation,” Hydrometallurgy, Vol. 99, No. (3–4), pp. 189–193.
Zhang, Q., Sugiyama, K., and Saito, F., 1997, “Enhancement of acid extraction of magnesium and silicon from serpentine by mechanochemical treatment,” Hydrometallurgy, Vol. 45, No. 3, pp. 323–331.
Zubryckyj, N., Evans, D.J.I., and Mackiw, V.N., 1965, “Preferential sulfation of nickel and cobalt in lateritic ores,” Journal of Metals, Vol. 17, pp. 478–486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, L., Rao, D.S., Mishra, B.K. et al. Characterization and dissolution of low-grade ferruginous nickel lateritic ore by sulfuric acid. Mining, Metallurgy & Exploration 31, 57–65 (2014). https://doi.org/10.1007/BF03402349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402349