Abstract
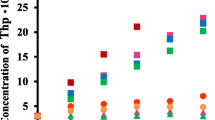
The stabilizing effects of α-, β-, γ- and dimethyl-β-cyclodextrins (α-, β-, γ-and DM-β-CyDs) on the degradation of hydrocortisone 17-butyrate (HC-17B) in aqueous solution was investigated. HC-17B underwent a facile hydroxide ion-catalyzed rearrangement to the less active 21-butyrate ester by the apparent first-order kinetics, and maximum stability of HC-17B was obtained at around, pH 4.0. The stability of HC-17B was increased by inclusion complexation with α-, γ- and DM-β-CyD in the pH range of 2.0–8.0 examined, whereas β-CyD accelerated the degradation of HC-17B at the pH higher than 5.0. The effects of ionic strength, solvent, temperature and CyD concentration were also investigated. Stability constants and apparent degradation rate constants of HC-17B-γ-CyD and HC-17B-DM-β-CyD complexes were determined kinetically on the basis of 1∶1 complexation. The results suggested that the inclusion complexation with γ-CyD or DM-β-CyD was most useful means to enhance the stability of the steroid.
Similar content being viewed by others
Literature Cited
Uekama K. Pharmaceutical applications of cyclodextrin complexations.Yakugaku Zasshi,101, 857 (1981).
Duchêne, D., Vaution, C. and Glomot, F.: Cyclodextrins, their value in pharmaceutical technology.Drug. Dev. Ind. Pharm. 12, 2193 (1986).
Uekama K.: Pharmaceutical applications of methylated cyclodextrins.Pharm. Int. March, 61 (1985).
O'Neil, R. C. and Carless, J. E.: Influence of side chain on the hydrolysis of some hydrocortisone esters.J. Pharm. Pharmacol. 31, 10P (1980).
Bundgaard, H. and Hansen, J.: Studies on the stability of corticosteroids. VI. Kinetics of the rearrangement of betamethasone-17-valerate to the 21-valerate ester in aqueous solution.Int. J. Pharm. 7, 197 (1981).
Anderson, B. D. and Taphouse, V.: Initial rate studies of hydrolysis and acyl migration in methylprednisolone-21-hemisuccinate and 17-hemisuccinate.J. Pharm. Sci. 70, 181 (1981).
Yip, Y. W. and Li Wan Po, A.: The stability of betamethasone-17-valerate in semi-solid bases.J. Pharm. Pharmacol. 31, 400 (1979).
Kametani T. Honda, T., Sato H. Suzuki M. and Nojiri, S.: Behavior of betamethasone-17-valerate in acidic or alkaline medium.Yakugaku Zasshi,101, 803 (1981).
Yip, Y. W., Li Wan Po A. and Irwin, W. J.: Kinetics of decomposition and formulation of hydrocortisone butyrate in semiaqueous and gel systems.J. Pharm. Sci. 72, 776 (1983).
Kawano Y., Ariga, A. and Shiba, M.: Stability and decomposition mechanism of hydrocortisone 17-butyrate.Yakuzaigaku,41, 71 (1981).
Chun, I. K. and Yun, D. S.: Inclusion complexations of hydrocortisone butyrate with various cyclodextrins in aqueous solution and in solid state.Int. J. Pharm. submitted (1992).
Olson, M. C.: Analysis of adrenocortical steroids in pharmaceutical preparations by high-pressure liquid chromatography.J. Pharm. Sci. 62, 2001 (1973).
Li Wan Po, A., Irwin, W. J. and Yip, Y. W.: High-performance liquid chromatographic assay of betamethasone 17-valerate and its degradation products.J. Chromatogr. 176, 399 (1976).
Andersen, F. M. and Bundgaard, H.: The influence of cyclodextrin complexation on the stability of betamethasone 17-valerate.Int. J. Pharm. 20, 155 (1984).
Tanaka, S., Uekama K. and Ikeda, K.: Hydrolysis rate of ethyl cinnamates in the presence of cyclodextrin.Chem. Pharm. Bull. 24, 2825 (1976).
Bender, M. L. and Komiyama, M.: “Cyclodextrin Chemistry”, Springer-Verlag, Berlin, 1978.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chun, I.K., Kim, B.Y. Influence of various cyclodextrins on the stability of hydrocortisone 17-butyrate in aqueous solution. Arch. Pharm. Res. 15, 176–183 (1992). https://doi.org/10.1007/BF02974095
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02974095