Abstract
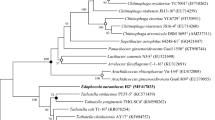
From a hydrothermal vent site off the Mexican west coast (20°50′N, 109°06′W) at a depth of 2,600 m, a novel, hyperthermophilic, anaerobic archaeum was isolated. Cells were round to slightly irregular cocci, 1.2–2.5 μm in diameter and were motile by means of a tuft of flagella. The new isolate grew between 60 and 93°C (optimum: 85°C), from pH 3.5 to 9 (optimum: pH 6.7), and from 0.8 to 8% NaCl (optimum: 2%). The isolate was an obligate organotroph, using chitin, yeast extract, meat extract, and peptone for growth. Chitin was fermented to H2, CO2, NH3, acetate, and formate. H2S was formed in the presence of sulfur. The chitinoclastic enzyme system was oxygen-stable, cell-associated, and inducible by chitin. The cell wall was composed of a surface layer of hexameric protein complexes arranged on a p6 lattice. The core lipids consisted of glycerol diphytanyl diethers and acyclic and cyclic glycerol diphytanyl tetraethers. The G+C content was 46.5 mol%. DNA/DNA hybridization and 16S rRNA sequencing indicated that the new isolate belongs to the genusThermococcus, representing a new species,Thermococcus chitonophagus. The type strain is isoalte GC74, DSM 10152.
Similar content being viewed by others
References
Adams MWW (1993) Enzymes and proteins from organisms that grow near and above 100°C. Annu Rev Microbiol 47:627–658
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth ofMethanobacterium ruminatium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Bassler BL, Yu C, Lee YC, Roseman S (1991) Chitin utilization by marine bacteria: degradation and catabolism of chitin oligosaccharides byVibrio furnissii. J Biol Chem 266:24276–24286
Baumeister W, Wildhaber J, Phipps BM (1989) Principles of organization in eubacterial and archaebacterial surface proteins, Can J Microbiol 35:215–227
Belkin S, Jannasch HW (1985) A new extremely thermophilic sulfur-reducing heterotrophic, marine bacterium. Arch Microbiol 141:181–186
Blöchl E, Burggraf S, Fiala G, Lauerer G, Huber G, Huber R, Rachel R, Segerer A, Stetter KO, Völkl P (1994) Isolation, taxonomy and phylogeny of hyperthermophilic microorganisms. World J Microbiol Biotechnol 11:1–8
Boyer JN (1986) End products of anaerobic chitin degradation by salt marsh bacteria as substrates for dissimilatory sulfate reduction and methanogenesis. Appl Environ Microbiol 52:1415–1418
Boyer JN (1994) Aerobic and anaerobic degradation and mineralization of14C-chitin by water column and sediment inocula of the York river estuary, Virginia. Appl Environ Microbiol 60: 174–179
Brenner DJ (1973) Desoxyribonucleic acid reassociation in the taxonomy of enteric bacteria. Int J Syst Bact 22:298–307
Brown SH, Kelly RM (1993) Characterization of amylolytic enzymes, having both α-1,4 and α-1,6 hydrolytic activity, from the thermophilic archaeaPyrococcus furiosus andThermococcus litoralis. Appl Environ Microbiol 59:2614–2621
Cabib E (1987) The synthesis and degradation of chitin. Adv Enzymol 59:59–101
Cavanaugh CM (1983) Symbiotic chemotrophic bacteria in marine invertebrates from sulfide-rich habitats. Nature 371:58–61
Davis B, Eveleigh DE (1984) Chitosanases: occurrence, production and immobilization. In: Zikakis JP (ed) Chitin, chitosan and related enzymes. Academic Press, Orlando, pp 161–179
Dworkin M, Reichenbach H (1981) The order Cytophagales (with addenda on the generaHerpetosiphon, Saprospira andFlexitrix). In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin Heidelberg New York, pp 374–376
Erauso G, Reysenbach AL, Godfroy A, Meunier JR, Crump B, Partensky F, Baross JA, Marteinsson V, Barbier G, Pace NR, Prieur D (1993)Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch Microbiol 160:338–349
Fiala G, Stetter KO (1986)Pyrococcus furiosus, sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol 145:56–61
Gage JD, Tyler PA (1991) Deep-sea hydrothermal vents and cold seeps. In: Gage JD, Tyler PA (eds) Deep-sea biology: a natural history of organisms at deep-sea floor. Cambridge University Press, Cambridge, pp 363–391
Gaill F, Hunt S (1986) Tubes of the deep-sea hydrothermal vent wormsRiftia pachyptila (Vestimentifera) andAlvinella pompejana (Annelida). Marit Ecol Program Ser 34:267–274
Gooday GW (1990) Physiology of microbial degradation of chitin and chitosan. Biodegradation 1:177–190
Gooday GW (1994) Physiology of microbial degradation of chitin and chitosan. In: Ratledge C (ed) Biochemistry of microbial degradation. Kluwer, Dordrecht, pp 279–312
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986)Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Huber R, Woese CR, Langworthy TA, Kristjansson JK, Stetter KO (1990)Fervidobacterium islandicum sp. nov., a new extremely thermophilic eubacterium belonging to the “Thermotogales”. Arch Microbiol 154:105–111
Huber R, Wilharm T, Huber D, Trincone A, Burggraf S, König H, Rachel R, Rockinger I, Fricke H, Stetter KO (1992)Aquifex pyrophilus gen. nov., sp. no., represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst Appl Microbiol 15:340–351
Huber H, Huber R, Lüdemann HD, Stetter KO (1994) Search for hyperthermophilic microorganisms in fluids obtained from the KTB pump test, Scientific Drill 4:127–129
Jannasch HW, Wirsen CO, Molyneaux SJ, Langworthy TA (1992) Comparative physiological studies on hyperthermophilic archea isolated from deep-sea hot vents with emphasis onPyrococcus strain GB-D. Appl Environ Microbiol 58:3472–3481
Jones ML (1980)Riftia pachyptila, new genus, new species, the vestimentiferan worm from the Galápagos rift geothermal vents (Pogonophora). Proc Biol Soc Wash 93:1295–1313
Jukes TH, Cantor GR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Kengen SWM, Stams AJM (1994) Formation of 1-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archaeonPyrococcus furiosus. Arch Microbiol 161:168–175
Kobayaschi T, Kwak YS, Akiba T, Kudo T, Horikoshi K (1994)Thermococcus profundus sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent, Syst Appl Microbiol 17:232–236
Koch R, Spreinat A, Lemke K, Antranikian G (1991) Purification and properties of a hyperthermoactive α-amylase from the archaeobacteriumPyrococcus furiosus. Arch Microbiol 155: 572–578
Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeck R, Macke TJ, Marsh TL, Woese CR (1993) The ribosomal database project. Nucleic Acids Res 21:3021–3023
Lauerer G, Kristjansson JK, Langworthy TA, König H, Stetter KO (1986)Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97°C. Syst Appl Microbiol 8:100–105
Ledl F, Schleicher E (1990) Die Maillard-Reaktion in Lebensmitteln und im menschlichen Körper—neue Ergebnisse zur Chemie, Biochemie und Medizin. Angew Chem 102:597–626
Leuschner C, Antranikian G (1994) Heat-stable enzymes from extremely thermophilic and hyperthermophilic microorganisms. World J Microbiol Biotechnol 11:95–114
Liaw HJ, Mah RA (1992) Isolation and characterization ofHaloanaerobacter chitinovorans gen nov., sp. nov., a halophilic, anaerobic, chitinolytic bacterium from solar saltern. Appl Environ Microbiol 58:260–266
Lonsdale P (1977) Clustering of suspension-feeding macrobenthos near abyssal hydrothermal vents at oceanic spreading centers. Deep-Sea Res 24:857–863
Lutz RA, Shank TM, Fornari DJ, Haymon RM, Lilley MD, Von Damm KL, Debruyers D (1994) Rapid growth at deep-sea vents. Nature 371:663–664
Marmur J (1961) A procedure for the isolation of desoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of desoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Meyer SA, Schleifer KH (1978) Desoxyribonucleic acid reassociation in the classification of coagulase-positive Staphylococci. Arch Microbiol 117:183–188
Miroshnichenko ML, Bonch-Osmolovskaya EA, Neuner A, Kostrikina NA, Chernych NA, Alekseev VA (1989)Thermococcus stetteri sp. nov., a new extremely thermophilic marine sulfur-metabolizing archaebacterium. Syst Appl Micribiol 12:257–262
Neuner A (1990) Isolierung, Charakterisierung und taxonomische Einordnung coccoider mariner hyperthermophiler Archaebakterien. PhD thesis, University of Regensburg, Germany
Neuner A, Jannasch HW, Belkin S, Stetter KO (1990)Thermococcus litoralis sp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch Microbiol 153:205–207
Pel R, Gottschal JC (1986) Mesophilic chitin-degrading anaerobes isolated from an estuarine environment. FEMS Microbiol Ecol 38:39–49
Pel R, Gottschal JC (1987) The effect of oxygen and sulfhydryl reagents on the hydrolysis and fermentation of chitin byClostridium 9.1. FEMS Microbiol Lett 44:59–62
Pel R, Hessels G, Aalfs H, Gottschal JC (1989) Chitin degradation byClostridium sp. strain 9.1 in mixed cultures with saccharolytic and sulfate-reducing bacteria. FEMS Microbiol Ecol 62:191–200
Rogers HJ (1961) The dissimilation of high molecular weight substances. In: Gunsalus IC, Stanier RY (eds) The bacteria. Academic Press. New York, pp 257–318
Saiki RK, Scharf SJ, Fallona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of b-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia, Science 230:1350–1354
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Schleifer KH, Stackebrandt E (1983) Molecular systematics of prokaryotes. Annu Rev Microbiol. 37:134–187
Simoneit BRT, Londsdale PF (1982) Hydrothermal petroleum in mineralized mounds at the seabed at Guaymas Basin. Nature 295:198–202
Stetter KO (1982) Ultrathin mycelia-forming organisms from submarine volcanic areas having an optimum growth temperature of 105°C. Nature 300:258–260
Stetter KO (1994) The lesson of archaebacteria. In: Bengtson S (ed) Nobel symposium no. 84. Columbia University Press, New York, pp 143–151
Stetter KO, König H, Stackebrandt E (1983)Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur-reducing archaebacteria growing optimally at 105°C. Syst Appl Microbiol 4:535–551
Stetter KO, Fiala G, Huber G, Huber R, Segerer A (1990) Hyper-thermophilic microorganisms. FEMS Microbiol Rev 75:117–124
Takayanagi T, Alisaka K, Takiguchi Y, Shimahara K (1991) Isolation and characterization of thermostable chitinases fromBacillus licheniformis X-7u. Biochim Biophys Acta 1078:404–410
Timmes K, Hobbs G, Berkley RCW (1974) Chitinolytic clostridia isolated from marine mud. Can J Microbiol 20:1284–1285
Tracey MV (1957) Chitin. Rev Pure Appl Chem 7:1–14
Trincone A, Lanzotti V, Nicolaus B, Zillig W, De Rosa M, Gambacorta A (1989) Comparative lipid composition of aerobically and anaerobically grownDesulfurolobus ambivalens, an autotrophic thermophilic archaeon. J Gen Microbiol 135:2751–2757
Tsujibo H, Minoura K, Miyamoto K, Endo H, Moriwaki M, Inamori Y (1993) Purification and properties of a thermostable chitinase fromStreptomyces thermoviolaceus OPC-520, Appl Environ Microbiol 59:620–622
Völkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter KO (1993)Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol 59:2918–2926
Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyaizu H, Yang D, Mandelco L, Sechrest J, Lawrence TG, Van Etten J, Maniloff J, Woese CR (1989) A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol 171:6455–6467
Wheelis ML, Kandler O, Woese CR (1992) On the nature of global classification. Proc Natl Acad Sci USA 89:2930–2934
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria and Eukarya. Proc Natl Acad Sci USA 87:4576–4579
Yang D, oyaizu Y, Oyaizu H, Olson GJ, Woese CR (1985) Mitochondrial origins. Proc Natl Acad Sci USA 82:4443–4447
Yu CH, Lee AM, Bassler BL, Roseman S (1991) Chitin utilization by marine bacteria: a physiological function for bacterial adhesion to immobilized carbohydrates. J Biol Chem 266:24260–24267
Zillig W, Holz I, Janekovic D, Schäfer W, Reiter WD (1983) The archaebacteriumThermococcus celer represents a novel genus within the thermophilic branch of the archaebacteria, Syst Appl Microbiol 4:88–94
Zillig W, Holz I, Klenk HP, Trent J, Wunderl S, Janekovic D, Imsel E, Haas B (1987)Pyrococcus woesei, sp. nov., an ultrathermophilic archaebacterium, representing a novel order, Thermococcales. Syst Appl Microbiol 9:62–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huber, R., Stöhr, J., Hohenhaus, S. et al. Thermococcus chitonophagus sp. nov., a novel, chitin-degrading, hyperthermophilic archaeum from a deep-sea hydrothermal vent environment. Arch. Microbiol. 164, 255–264 (1995). https://doi.org/10.1007/BF02529959
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02529959