Abstract
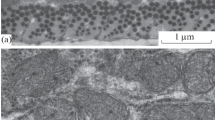
Electron microscopy shows that intact mitochondria can be isolated from neck-muscle stored at 144h post-mortem at 4°. Isolated mitochondria, all in the condensed configuration, have clearly defined outer and inner membranes, outer compartments and intracristal spaces; a larger proportion of swollen ones was isolated from the 144h than from the 120 h post-mortem tissue.
Mitochondria from 96 h tissue still retained the following % of the initial values for the ADP/O ratio, respiratory control index (RCI) and state 3 respiratory rate observed for 0–5h tissue: malate+pyruvate, 100, 72 and 53; succinate, 80, 30 and 74; ascorbate+ tetramethyl-p-phenylencdiamine (TMPD), 92, 88 and 72.
Both the succinate and ascorbate-TMPD oxidase systems appear to have a “critical” storage time of about 70 h, whereas the malate+pyruvate system has one of about 96 h. Asharp decline of the ADP/O ratio, RCI and the state 3 respiratory rate occurred after this time, but these three parameters were better preserved in the ascorbate-TMPD oxidase system.
The oxidation of the citric acid cycle intermediates in the neck-muscle mitochondria thus shows a higher sensitivity to post-mortem ageing with respect to cytochrome oxidase activity. This is probably due to post-mortem muscle acidification.
Similar content being viewed by others
References
D. Greiff and M. Myers,Biochim. Biophys. Acta. 78 (1963) 45.
R. G. Walton, M. Kervina, S. Fleicher and D. S. Dow,J. Bioenergetics,1 (1970) 3.
E. Carafoli and P. Gazzotti,Biochem. Biophys. Res. Commun.,39 (1970) 842.
K. Ozawa, K. Seta, H. Takeda, K. Ando, H. Handa and C. Araki,J. Biochem. (Tokyo),59 (1966) 501.
I. Boime, E. E. Smith and F. E. Hunter, Jr.,Arch. Biochem. Biophys.,139 (1970) 425.
I. Boime, E. E. Smith and F. E. Hunter, Jr.,Arch. Biochem. Biophys. 128 (1968) 704.
K. S. Cheah,FERS letters,10 (1970) 109.
J. D. Luft,J. Biophys. Biochem. Cytol.,9 (1961) 409.
B. Chance and G. R. Williams, in:Advances in Enzymology, F. F. Nord (ed.), Vol. 17, Interscience Publishers, Inc., New York, 1956, p. 65.
O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall,J. Biol. Chem.,193 (1951) 265.
C. Hackenbrock,J. Cell Biol.,30 (1966) 269.
J. R. Bendall and R. A. Lawrie,Animal Breed. Abstr.,32 (1964) 1.
B. Chance and H. ConradJ. Biol. Chem.,234 (1959) 1568.
D. D. Tyler, R. W. Estabrook and D. R. Sanadi,Arch. Biochem. Biophys.,114 (1966) 239.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cheah, K.S., Cheah, A.M. Post-mortem changes in structure and function of ox muscle mitochondria. 1. Electron microscopic and polarographic investigations. J Bioenerg Biomembr 2, 85–92 (1971). https://doi.org/10.1007/BF01648923
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01648923