Abstract
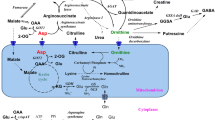
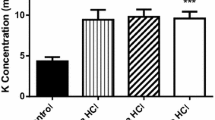
Intravenous infusion of 0.5 mmol/kgl-lysine monohydrochloride was performed in six normal volunteer subjects aged 10–14 years to study the inhibitory effect of lysine on urea cycle metabolism. The lysine infusion resulted in a significant increase in plasma levels of arginine and ornithine, and in urinary homocitrulline, putrescine, and orotic acid, accompanied by a significant increase in blood ammonia. There was little change in plasma urea and citrulline. The increase in plasma arginine appears to reflect an inhibited arginase activity although the plasma urea level did not change. The increased homocitrulline excretion suggests that ornithine conversion to citrulline via ornithine transcarbamylase (OTC) was inhibited. The simultaneous increase in plasma ornithine and urinary putrescine may reflect an inhibition of mitochondrial ornithine transport. In addition to the direct ammoniagenic property of lysine, impaired ornithine conversion to citrulline resulting from the inhibition of both OTC activity and mitochondrial ornithine uptake by lysine may be responsible for the increase in blood ammonia and urinary orotic acid. Despite the retarded citrulline formation, a promoted efflux of citrulline from mitochondria may have kept the plasma citrulline level unchanged.
Similar content being viewed by others
Abbreviations
- OTC:
-
ornithine transcarbamylase
References
Batshaw ML, Walser M, Brusilow SW (1980) Plasma α-ketoglutarate in urea cycle enzymopathies and its role as a harbinger of hyperammonemic coma. Pediatr Res 14:1316–1319
Bradford NM, McGivan JD (1980) Evidence for the existence of an ornithine/citrulline antiporter in rat liver mitochondria. FEBS Lett 113:294–298
Cederbaum SD, Shaw KNF, Spector EB, Verity MA, Snodgrass PJ, Sugarman GI (1979) Hyperargininemia with arginase deficiency. Pediatr Res 13:827–833
Cittadini D, Pietropaolo C, de Cristofaro D, D'Ayjello-Caracciolo M (1964) In vivo effect ofL-lysine on rat liver arginase. Nature 203:643–644
de Vrese M, Barth CA (1985) Influence of lysine on urea cycle activity and orotate formation in the isolated perfused rat liver. Biol Chem Hoppe-Seyler 366:455–461
Fico ME, Hassan AS, Milner JA (1982) The influence of excess lysine on urea cycle operation and pyrimidine biosynthesis. J Nutr 112:1854–1861
Freedland RA, Crozier GL, Hicks BL, Meijer AJ (1984) Arginine uptake by isolated rat liver mitochondria. Biochim Biophys Acta 802:407–412
Hallett CJ, Cook JGH (1971) Reduced nicotinamide adenine dinucleotide-coupled reaction for emergency blood urea estimation. Clin Chim Acta 35:33–37
Hommes FA, Kitchings L, Eller AG (1983) The uptake of ornithine and lysine by rat liver mitochondria. Biochem Med 30: 313–321
Lusty CJ, Jilka RL, Nietsch EH (1979) Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem 254:10030–10036
Marton LJ, Russell DH, Levy CC (1973) Measurement of putrescine, spermidine, and spermine in physiological fluids by use of an amino acid analyzer. Clin Chem 19:923–926
Persson LO (1981) Decarboxylation of ornithine and lysine by ornithine decarboxylase from kidneys of testosterone treated mice. Acta Chem Scand [B] 35:451–459
Ratnaike RN, Buttery JE, Hoffmann S (1984) Blood ammonia measurement using a simple reflectometer. J Clin Chem Clin Biochem 22:105–108
Rogers LE, Porter FS (1968) Hereditary orotic aciduria. 11. A urinary screening test. Pediatrics 42:423–428
Segal S, Thier SO (1983) Cystinuria. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Brown MS (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 1774–1791
Shih VE (1978) Urea cycle disorders and other congenital hyperammonemic syndromes. In: Stanbury JB, Wyngaarden JB, Fredrickson DS (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 362–386
Simell O, Luukkainen P, Sipilä I (1985) Alanine-induced hyper-ammonemia in hyperlysinemia and in saccharopinuria. Pediatr Res 19:320A
Statter M, Russell A (1978) Competitive interrelationships between lysine and arginine in rat liver under normal conditions and in experimental hyperammonemia. Life Sci 22:2097–2102
Ulman EA, Kari FW, Hevia P, Visek WJ (1981) Orotic aciduria caused by feeding excess lysine to growing rats. J Nutr 111:1772–1779
Valle D, Simell O (1983) The hyperornithinemias. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Brown MS (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 382–401
Zweig JI (1973) Effects of lysine on ammonia formation, hydrogen ion, and potassium ion balance: A review and an hypothesis. Clin Chem 19:943–949
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kato, T., Sano, M. & Mizutani, N. Inhibitory effect of intravenous lysine infusion on urea cycle metabolism. Eur J Pediatr 146, 56–58 (1987). https://doi.org/10.1007/BF00647285
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00647285