Summary
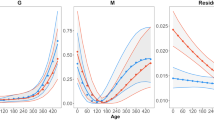
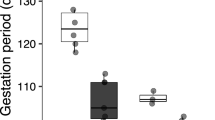
Hatchling Sceloporus occidentalis from northern populations (central Oregon) grow more slowly than hatchlings from southern populations (southern California) in nature. In this study, I determine whether this difference in growth rate results from differences in thermal environment and/or in thermoregulatory behavior. To determine the degree to which the thermal environment affects growth rate among populations, I reared hatchings from the northern and southern populations in a cycling thermal regime in one of three experimental treatments differing in access to radiant heat (6, 9, or 12 h radiant heat; remainder of 24 h at 15°C). I also measured the body temperature that each individual voluntarily selected over the course of the daily activity cycle. Growth rate varied positively with duration of access to radiant heat. Within the three treatments, individual growth rate was positively correlated with body temperature. Moreover, the difference in growth rate between the northern and southern populations was due in part to differences in behavior — individuals from northern populations selected lower body temperatures. I found that significant variation in body temperature was associated with family membership, suggesting that thermal physiology has a genetic basis. Moreover, growth rate was correlated with body temperature among families in each population suggesting a genetic correlation underlies the phenotypic correlations. Thus, genetically based variation in thermal physiology contributes to differences in growth rate among individuals within a population as well as to differences among populations.
Similar content being viewed by others
References
Adolph SC (1987) Physiological and behavioral ecology of the lizards Sceloporus occidentalis and Sceloporus graciosus. Dissertation, University of Washington, Seattle, WA
Adolph SC (1990) Influence of behavioral thermoregulatio on microhabitat use by two Sceloporus lizards. Ecology 71: 315–327
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the reptilia vol 13. Academic Press, New York, pp 273–320
Arnold SJ (1981) Behavioral variation in natural populations. I. Phenotypic, genetic and environmental correlations betwen chemoreceptive responses to prey in the garter snake, Thamnophis elegans. Evolution 35: 489–509
Arnold SJ (1987) Genetic correlation and the evolution of physiology. In: Feder ME, Bennett AF, Burggren WW, Huey RB (eds) New directions in ecological physiology, Cambridge University Press, Cambridge, pp 189–211
Arnold SJ, Bennett AF (1984) Behavioural variation in natural populations. III. Antipredator displays in the garter snake Thamnophis radix. Anim Behav 32: 1108–1118
Avery RA (1973) Morphometric and functional studies on the stomach of the lizard Lacerta vivipara. J Zool (London) 169: 157–167
Avery RA (1979) Lizards — A Study in Thermoregulation. University Park Press, Baltimore
Avery RA (1984) Physiological aspects of lizard growth: the role of thermoregulation. Symp Zool Soc Lond 52: 407–424
Ballinger RE (1977) Reproductive strategies: food availability as a source of proximal variation in lizard. Ecology 58: 628–635
Ballinger RE (1983) Life-history variations: In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: studies of a model organism, Harvard Univ Press, Cambridge, MA pp 241–260
Ballinger RE, Congdon JD (1980) Food resource limitation of body growth rates in Sceloporus scalaris (Sauria: Iguanidae). Copeia 1980: 921–923
Beitinger TL, Fitzpatrick LC (1979) Physiological and ecological correlates of preferred temperature in fish. Am Zool 19: 319–329
Bennett AF, Gleeson TT (1976) Activity metabolism in the lizard Sceloporus occidentalis. Physiol Zool 49: 65–76
Berkum FH van, Tsuji JS (1987) Inter-familial differences in sprit speed of hatchling lizards (Sceloporus occidentalis). J Zool (London) 212: 511–519
Berven KA, Gill DE,(1983) Interpreting geographic variation in life-history traits. Am Zool 23: 85–97
Berven KA, Gill DE, Smith-Gill SJ (1979) Countergradient selection in the green frog, Rana clamitans. Evolution 33: 609–623
Brattstrom BH (1974) Energetic responses of salmon to temperature: A study of some thermal relations in the physiology and freshwater ecology of Sockeye Salmon (Oncorrhynchus nerka). Am Zool 11: 99–113
Buffenstein R, Louw G (1982) Temperature effects on bioenergetics of growth, assimilation efficiency and thyroid activity in juvenile varanid lizards. J Therm Biol 7: 197–200
Case TJ (1978) On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q Rev Biol 53: 243–282
Christian KA (1986) Physiological consequences of night-time temperature for a tropical herbivorous lizard (Cydura nubila). Can J Zool 64: 836–846
Christian KA, Tracy CR (1984) Physiological and ecological consequences of sleeping-site selection by the Galapagos land iguanas (Conolophus pallidus). Ecology 65: 752–758
Dawson WR (1975) On the physiological significance of the preferred temperatures of reptiles. In: Gates DM, Schmerl RB (eds) Perspectives in Biophysical Ecology. Ecological Studies, vol 12, pp 443–473. Springer, Berlin Heidelberg New York
Dewitt CB, Friedman RM (1976) The significance of skewness in ectotherm thermoregulation. Am Zool 19: 195–209
Dunham AE (1978) Food availability as a proximate factor influencing individual growth rates in the iguanid lizard Sceloporus merriam. Ecology 52: 770–778
Duham AE, Grant BW, Overall KL (1989) Interfaces between biophysical and physiological ecology and the population biology of terrestrial vertebrate ectotherms. Physiol Zool 62: 335–355
Falconer DW (1981) Introduction to quantitative genetics. 2nd ed Longman, Great Britain
Ferguson GW, Fox SF (1984) Annual variation of survival advantage of large juvenile side-blotched lizards, Uta stansburiana: its causes and evolutionary significance. Evolution 38: 342–349
Ferner J (1974) Home-range size and overlap in Sceloporus undulatus erythrocheilus (Reptilia: Iguanidae). Copeia 1974: 332–337
Fox SF (1978) Natural selection on behavioral phenotypes of the lizard Uta stansburiana. Ecology 59: 834–847
Fox SF (1983) Fitness, home-range quality, and aggression in Uta stansburiana. In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: Studies of a model organism, pp 149–168, Harvard Univ Press, Cambridge, MA
Fox SF, Rose E, Myers R (1981) Dominance and the acquisition of superior home ranges in the lizard Uta stansburiana. Ecology 62: 888–893
Gadgil M, Bossert WH (1970) Life historical consequences of natural selection. Am Natur 104: 1–24
Grant BW, Dunham AE (1988) Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 69: 167–176
Harwood RH (1979) The effect of temperature on the digestive efficiency of three species of lizards, Cnemidophorus tigris, Gerrhonotus multicarinatus and Sceloporus occidentalis. Comp Biochem Phys 63A: 417–433
Hutchinson VH, Maness JH (1979) The role of behavior in temperature acclimation and tolerance in ectotherms. Am Zool 19: 367–384
Licht P (1967) Thermal adaptation in the enzymes of lizards in relation to preferred body temperatures. In: Molecular mechanims of temperature adaptation, American Association for the Advancement of Science, Washington, DC, USA 131–145
Lillywhite HB, Licht P, Chelgren P (1973) The role of behavioral thermoregulation in the growth energetics of the toad, Bufo boreas. Ecology 54: 375–383
McGinnis SM (1966) Sceloporus occidentalis: Preferred body temperature of the western fence lizard. Science 152: 1090–1091
Parker WS, Pianka ER (1975) Comparative ecology of populations of the lizard Uta stansburiana. Copeia 1975: 615–632
Pianka ER (1970) Comparative autecology of the lizard Cnemidophorus tigris in different parts of its geographic range. Ecology 51: 703–720
Porter WP (1989) New animal models and experiments for calculating growth potential at different elevations. Physiol Zool 62: 286–313
Porter WP, Tracy CR (1983) Biophysical analyses of energetics, time-space utilization, and distributional limits. In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: studies of a model organism, pp 55–83. Harvard Univ Press, Cambridge, MA
Rand AS (1967) Ecology and social organization in the iguanid lizard, Anolis lineatopus. Proceedings of United States National Museum 122: 1–79
Regal PJ (1967) Voluntary hypothermia in reptiles. Science 155: 1551–1553
Rose B (1981) Factors affecting activity in Sceloporus varigatus. Ecology 62: 706–716
Ruby DE (1981) Phenotypic correlates of male reproductive success in the lizard, Sceloporus jarrovi. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior, pp 96–107. Chiron Press, New York
Ruby DE (1984) Male breeding success and differential access to females in Anolis carolinensis. Herpetologica 40: 272–280
Ruth SB (1977) A comparison of the demography and female reproduction in sympatric western fence lizards (Sceloporus occidentalis) and sage-brush lizards (Sceloporus graciosus) on Mount Diablo, California. Dissertation, University of California, Berkeley, CA, USA
Sinervo B (1990) The evolution of maternal investment in lizards: an experimental and comparative analysis of egg size and its effects on offspring performance. Evolution (in press)
Sinervo B, Adolph SC (1989) Thermal sensitivity of hatchling growth in Sceloporus lizards: environmental, behavioral and genetic aspects. Oecologia 78: 411–419
Skoczylas R (1978) Physiology of the digestive tract. In: Gans C, Gans KA (eds) Biology of the reptilia, vol 8, 589–717. Academic Press, New York
Stamps JA (1983) The relationship between ontogentic habitat shifts, competition and predator avoidance in a juvenile lizard (Anolis aeneus). Behav Ecol Sociobiol 15: 115–119
Stamps JA (1984) Rank-dependent compromises between growth and predator protection in lizard dominance hierarchies. Anim Behav 32: 1101–1107
Stamps JA, Tanaka S (1981) The influence of food and water on growth rates in a tropical lizard (Anolis aeneus). Ecology 62: 33–40
Stearns SC (1983) The evolution of life-history traits in mosquito fish since their introduction to Hawaii in 1905: rates of evolution, heritabilities, and developmental plasticity. Am Zool 3323: 65–75
Stinner RE, Gutierrez AP, Butler GD (1974) An algorthim for temperature dependent growth rate simulation. Can Entomol 106: 519–524
Taylor F (1979) Convergence to the stable age distribution in population of insects. Am Nat 113: 511–530
Taylor F (1981) Ecology and evolution of physiological time in insects. Am Nat 117: 1–23
Tinkle DW (1967) The life and demography of the side-blotched lizard, Uta stansburiana. Misc Publ Mus Zool Univ Michigan 132: 1–182
Tinkle DW (1969) Evolutionary implications of comparative population studies in the lizard Uta stansburiana. In: Systematic Biology. Proceedings of an International Conference on Systematic Biology, Publication 1962, National Academy of Sciences, Washington, DC, USA 133–154
Tinkle DW (1972) The role of environment in the evolution of life history differences within and between lizard species. Univ Ark Mus Occas Pap 4: 77–100
Tinkle DW, Ballinger RE (1972) Sceloporus undulatus: a study of the intraspecific comparative demography of a lizard. Ecology 53: 570–584
Tinkle DW, Wilbur HM, Tilley GS (1970) Evolutionary strategies in lizard reproduction. Evolution 24: 55–74
Tracy CR (1980) Water relations of parchement-shelled lizard (Sceloporus undulatus) eggs. Copeia 1980: 478–482
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual Selecton and Descent of Man, pp 136–179. Aldine, Chicago
Tsuji JS (1988a) Seasonal profiles of standard metabolic rate of lizards (Sceloporus occidentalis) in relation to latitude. Physiol Zool 61: 230–240
Tsuji JS (1988b) Thermal acclimation of metabolism in Sceloporus from different latitudes. Physiol Zool 61: 241–253
Tsuji J, Huey RB, van Berkum FH, Garland T Jr, Shaw R (1989) Locomotor performance of hatchling fence lizards (Sceloporus occidentalis): quantitative genetics and morphological correlates. Evol Ecol 3: 240–255
Waldschmidt SR, Jones SM, Porter WP (1986) The effect of body temperature and feeding regime on activity, passage time, and digestive coefficient in the lizard Uta stansburiana. Physiol Zool 59: 376–383
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sinervo, B. Evolution of thermal physiology and growth rate between populations of the western fence lizard (Sceloporus occidentalis). Oecologia 83, 228–237 (1990). https://doi.org/10.1007/BF00317757
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317757