Abstract
Purpose of Review
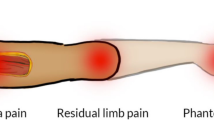
A considerable number of major limb amputation patients will develop symptomatic neuromas within the residual limb. There has been a paradigm shift in the surgical treatment of symptomatic neuromas from techniques that attempt to interrupt axonal regeneration in favor of techniques that permit reinnervation. In addition, growing evidence suggests that successful treatment of peripheral nerve pain may diminish the development of phantom limb pain. We discuss novel surgical techniques that seek to prevent neuroma formation and in turn reduce phantom limb pain.
Recent Findings
Targeted muscle reinnervation and regenerative peripheral nerve interface surgery provide regenerating peripheral nerves denervated targets for reinnervation thus reducing the number of free axons available to form neuromas. While very different in technique, both approaches decrease the formation of symptomatic neuromas. In doing so, these methods reduce the experience of residual limb pain and phantom limb pain.
Summary
Implementation of innovative surgical techniques after peripheral nerve transection can transform the management of postamputation pain. Decreasing the impact of neuroma pain and phantom limb pain will facilitate prosthetic rehabilitation, decrease analgesic use, and improve quality of life after limb amputation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9.
Pinzur MS, Gottschalk F, Pinto MA, Smith DG. Controversies in lower extremity amputation. Instr Course Lect. 2008;57:663–72.
• Dellon AL, Aszmann OC. In musculus, veritas? Nerve “in muscle” versus targeted muscle reinnervation versus regenerative peripheral nerve interface: historical review. Microsurgery. 2020. This editorial reviewed the historical treatment of neuromas and compared the novel techniques targeted muscle reinnervation and regenerative peripheral nerve interface.
Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–36.
Smith DG, Ehde DM, Legro MW, Reiber GE, del Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;361:29–38.
McFarland LV, Hubbard Winkler SL, Heinemann AW, Jones M, Esquenazi A. Unilateral upper-limb loss: satisfaction and prosthetic-device use in veterans and servicemembers from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47(4):299–316.
Stokvis A, Ruijs AC, van Neck JW, Coert JH. Cold intolerance in surgically treated neuroma patients: a prospective follow-up study. J Hand Surg [Am]. 2009;34(9):1689–95.
van der Avoort DJ, Hovius SE, Selles RW, van Neck JW, Coert JH. The incidence of symptomatic neuroma in amputation and neurorrhaphy patients. J Plast Reconstr Aesthet Surg. 2013;66(10):1330–4.
Watson J, Gonzalez M, Romero A, Kerns J. Neuromas of the hand and upper extremity. J Hand Surg [Am]. 2010;35(3):499–510.
Dworkin RH, Handlin DS, Richlin DM, Brand L, Vannucci C. Unraveling the effects of compensation, litigation, and employment on treatment response in chronic pain. Pain. 1985;23(1):49–59.
Harden RN. Chronic neuropathic pain. Mechanisms, diagnosis, and treatment. Neurologist. 2005;11(2):111–22.
Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862–9.
Wolvetang NHA, Lans J, Verhiel SHWL, Notermans BJW, Chen NC, Eberlin KR. Surgery for symptomatic neuroma: anatomic distribution and predictors of secondary surgery. Plast Reconstr Surg. 2019;143(6):1762–71.
•• Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4(12):e1038 This retrospective paper presents the first case series of patients who underwent RPNI for the treatment of symptomatic neuromas. Results showed that patients had 71% reduction in neuroma pain and 53% reduction in phantom pain.
Kumar N, Stevenson JH. Intractable digital neuroma pain; the ultimate solution? Br J Plast Surg. 1990;43(1):122–3.
Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62–7 discussion 7-8.
Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H. Treatments of traumatic neuropathic pain: a systematic review. Oncotarget. 2017;8(34):57670–9.
(NIS) HNIS. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2009.
Davis RW. Phantom sensation, phantom pain, and stump pain. Arch Phys Med Rehabil. 1993;74(1):79–91.
• Kubiak CA, Kemp SWP, Cederna PS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153(7):681–2 The authors presented the RPNI technique while discussing the innovative nature of the procedure and presented the advantages of RPNI over previous neuroma treatment techniques. The barriers to use, potential effects on clinical care and benefits of the treatment were discussed.
Luo Y, Anderson TA. Phantom limb pain: a review. Int Anesthesiol Clin. 2016;54(2):121–39.
Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21(3):267–78.
Jensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17(3):243–56.
Shukla GD, Sahu SC, Tripathi RP, Gupta DK. Phantom limb: a phenomenological study. Br J Psychiatry. 1982;141:54–8.
Sherman RA, Sherman CJ, Parker L. Chronic phantom and stump pain among American veterans: results of a survey. Pain. 1984;18(1):83–95.
Kim PS, Ko JH, O'Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg [Am]. 2012;37(8):1609–16.
Pierrie SN, Gaston RG, Loeffler BJ. Targeted muscle reinnervation for prosthesis optimization and neuroma management in the setting of transradial amputation. J Hand Surg [Am]. 2019;44(6):525.e1–8.
Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472(10):2984–90.
•• Kubiak CA, SWP K, Cederna PS, Kung TA. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg. 2019;144(3):421e–30e A retrospective review compared 45 patients who underwent RPNI at the time of primary amputation to 45 control patients who underwent amputation without RPNI to determine if RPNIs prevented the formation of symptomatic neuromas and lessened phantom limb pain. The RPNI group had a significantly lower incidence of symptomatic neuromas and phantom limb pain.
• Harte S, Harris R, Clauw D. The neurobiology of central sensitization. J Appl Biobehav Res. 2018;23:e12137 The authors describe the neurobiological phenomenon of central sensitization and its role in chronic pain conditions.
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926.
Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95–106.
Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7(1 Suppl 1):S3–S12.
Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974;43(3):580–93.
Flor H, Nikolajsen L, Staehelin JT. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7(11):873–81.
Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355(6355):75–8.
Flor H, Elbert T, Mühlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 1998;119(2):205–12.
Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15(12):8083–95.
Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109(1–2):57–77.
Nyström B, Hagbarth KE. Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neurosci Lett. 1981;27(2):211–6.
•• Poppler LH, Parikh RP, Bichanich MJ, Rebehn K, Bettlach CR, Mackinnon SE, et al. Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain. 2018;159(2):214–23 This meta-analysis reviewed 54 studies to evaluate the role of surgical technique on the outcome of surgical management of symptomatic neuromas. They found that surgical treatment was effective in 77% of patients with no significant differences between techniques. The review did not include TMR and RPNI techniques in the comparisons.
Herndon JH, Eaton RG, Littler JW. Management of painful neuromas in the hand. J Bone Joint Surg Am. 1976;58(3):369–73.
Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121(3):908–14 discussion 15-7.
Devor M, Govrin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22(2):127–37.
Korenman EM, Devor M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp Neurol. 1981;72(1):63–81.
Meyer RA, Raja SN, Campbell JN, Mackinnon SE, Dellon AL. Neural activity originating from a neuroma in the baboon. Brain Res. 1985;325(1–2):255–60.
Elliot D. Surgical management of painful peripheral nerves. Clin Plast Surg. 2014;41(3):589–613.
Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg [Am]. 1977;2(1):70–8.
Tupper JW, Booth DM. Treatment of painful neuromas of sensory nerves in the hand: a comparison of traditional and newer methods. J Hand Surg [Am]. 1976;1(2):144–51.
Brandner MD, Buncke HJ, Campagna-Pinto D. Experimental treatment of neuromas in the rat by retrograde axoplasmic transport of ricin with selective destruction of ganglion cells. J Hand Surg [Am]. 1989;14(4):710–4.
Nennesmo I, Kristensson K. Effects of retrograde axonal transport of Ricinus communis agglutinin I on neuroma formation. Acta Neuropathol. 1986;70(3–4):279–83.
Shapiro S, Voelker J. Reduction of experimental neuroma formation with ricin. J Surg Res. 1991;51(5):405–8.
Zur ML Behandlung der schmerzhaften neurome. Zmtralbl. Clair; 1918. p. 547.
Dellon AL, Mackinnon SE, Pestronk A. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg. 1984;12(1):30–40.
Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg. 1985;76(3):345–53.
Barberá J, Albert-Pampló R. Centrocentral anastomosis of the proximal nerve stump in the treatment of painful amputation neuromas of major nerves. J Neurosurg. 1993;79(3):331–4.
Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetics Orthot Int. 2004;28(3):245–53.
O'Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am. 2008;90(2):393–400.
Mavrogenis AF, Pavlakis K, Stamatoukou A, Papagelopoulos PJ, Theoharis S, Zoubos AB, et al. Current treatment concepts for neuromas-in-continuity. Injury. 2008;39(Suppl 3):S43–8.
•• Dumanian GA, Potter BK, Mioton LM, Ko JH, Cheesborough JE, Souza JM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270(2):238–46 This randomized clinical trial compared 28 patients who were randomized to TMR or “standard treatment” (neurom excision and burying into muscle) for the treatment of postamputation pain. The TMR cohort had improved phantom limb pain and trended toward improved residual limb pain.
•• Valerio IL, Dumanian GA, Jordan SW, Mioton LM, Bowen JB, West JM, et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228(3):217–26 This cohort study compared 51 patients undergoing major limb amputation with immediate TMR to 438 patients undergoing major limb amputation without TMR to determine if TMR at the time of amputation decreases phantom limb pain and residual limb pain severity. The TMR cohort has less phantom limb pain and residual limb pain.
Urbanchek MG, Kung TA, Frost CM, Martin DC, Larkin LM, Wollstein A, et al. Development of a regenerative peripheral nerve interface for control of a neuroprosthetic limb. Biomed Res Int. 2016;2016:5726730.
Woo SL, Urbanchek MG, Cederna PS, Langhals NB. Revisiting nonvascularized partial muscle grafts: a novel use for prosthetic control. Plast Reconstr Surg. 2014;134(2):344e–6e.
Carlson BM, Gutmann E. Regneration in free grafts of normal and denervated muscles in the rat: morphology and histochemistry. Anat Rec. 1975;183(1):47–62.
Gart MS, Souza JM, Dumanian GA. Targeted muscle Reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg [Am]. 2015;40(9):1877–88.
Morgan EN, Kyle Potter B, Souza JM, Tintle SM, Nanos GP. Targeted muscle reinnervation for transradial amputation: description of operative technique. Tech Hand Up Extrem Surg. 2016;20(4):166–71.
Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133(6):1380–94.
Urbanchek M, Baghmanli Z, Moon J, Sugg K, Langhals N, Cederna P. Quantification of regenerative peripheral nerve interface signal transmission. Plast Reconstr Surg. 2012;130:55–6.
Bader D. Reinnervation of motor endplate-containing and motor endplate-less muscle grafts. Dev Biol. 1980;77(2):315–27.
Hakelius L, Nyström B, Stålberg E. Histochemical and neurophysiological studies of autotransplanted cat muscle. Scand J Plast Reconstr Surg. 1975;9(1):15–24.
Killer H, Müntener M. Time course of the regeneration of the endplate zone after autologous muscle transplantation. Experientia. 1986;42(3):301–2.
Dellon AL. Muscle sense, or nonsense? Ann Plast Surg. 1991;26(5):444–8.
Dellon AL, Witebsky FG, Terrill RE. The denervated Meissner corpuscle. A sequential histological study after nerve division in the Rhesus monkey. Plast Reconstr Surg. 1975;56(2):182–93.
Dellon AL. Reinnervation of denervated Meissner corpuscles: a sequential histologic study in the monkey following fasicular nerve repair. J Hand Surg [Am]. 1976;1(2):98–109.
Kuiken TA, Marasco PD, Lock BA, Harden RN, Dewald JP. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci U S A. 2007;104(50):20061–6.
Banks RW, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol. 1989;408:345–72.
Banks RW. The innervation of the muscle spindle: a personal history. J Anat. 2015;227(2):115–35.
Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103(2):503–10.
Bain JR, Hason Y, Veltri K, Fahnestock M, Quartly C. Clinical application of sensory protection of denervated muscle. J Neurosurg. 2008;109(5):955–61.
Hynes NM, Bain JR, Thoma A, Veltri K, Maguire JA. Preservation of denervated muscle by sensory protection in rats. J Reconstr Microsurg. 1997;13(5):337–43.
•• Vu PP, Vaskov AK, Irwin ZT, Henning PT, Lueders DR, Laidlaw AT, et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci Transl Med. 2020;12(533):eaay2857 The ability of the RPNI to serve as an amplifier of motor action potentials with stability in upper limb amputation patients was shown in this research article. RPNI interfaces showed contraction during phantom finger flexion confirming the functional reinnveration of the interfaces in two patients. Patients successfully controlled a hand prosthesis using the RPNI signals.
Hooper R, Cederna P, Brown D, Haase S, Waljee J, Egeland B, et al. Regenerative peripheral nerve interface for the management of symptomatic hand and digital neuromas. Plastic and Reconstructive Surgery Global Open 2020.
Coluzzi F, Bifulco F, Cuomo A, Dauri M, Leonardi C, Melotti RM, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017;13:1163–73.
Chapman CR, Donaldson G, Davis J, Ericson D, Billharz J. Postoperative pain patterns in chronic pain patients: a pilot study. Pain Med. 2009;10(3):481–7.
Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Phys Med Rehabil Clin N Am. 2006;17(2):287–302.
Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039–44.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7.
de Heer EW, Gerrits MM, Beekman AT, Dekker J, van Marwijk HW, de Waal MW, et al. The association of depression and anxiety with pain: a study from NESDA. PLoS One. 2014;9(10):e106907.
Scott EL, Kroenke K, Wu J, Yu Z. Beneficial effects of improvement in depression, pain catastrophizing, and anxiety on pain outcomes: a 12-month longitudinal analysis. J Pain. 2016;17(2):215–22.
Sullivan M, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess. 1995;7(4):524–32.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Amputation Rehabilitation
Rights and permissions
About this article
Cite this article
Hart, S.E., Kung, T.A. Novel Approaches to Reduce Symptomatic Neuroma Pain After Limb Amputation. Curr Phys Med Rehabil Rep 8, 83–91 (2020). https://doi.org/10.1007/s40141-020-00276-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-020-00276-2