Abstract
Purpose
Recent data have shown a decreasing overall mortality in acromegaly over the last decades. However, cancer incidence and cancer-related mortality still appear to be increased. Our aim was to obtain updated epidemiological data from Norway in a clinically well-defined cohort with complete register-based follow-up.
Methods
Patients diagnosed with acromegaly from South-Eastern Norway between 1999–2019 (n = 262) and age and sex matched population controls (1:100) were included (n = 26,200). Mortality and cancer data were obtained from the Norwegian Cause of Death and Cancer Registry. Mortality and cancer incidence were compared by Kaplan–Meier analyses and Cox regression; we report hazard ratios (HRs) with 95% confidence intervals (95% CI).
Results
Median age at diagnosis was 48.0 years (interquartile range (IQR): 37.6–58.0). Mean annual acromegaly incidence rate was 4.7 (95% CI 4.2–5.3) cases/106 person-years, and the point prevalence (2019) was 83 (95% CI 72.6–93.5) cases/106 persons. Overall mortality was not increased in acromegaly, HR 0.8 (95% CI 0.5–1.4), cancer-specific and cardiovascular-specific mortality was also not increased (HR: 0.7 (95% CI 0.3–1.8) and 0.8 (95% CI: 0.3–2.5) respectively). The HR for all cancers was 1.45 (1.0–2.1; p = 0.052).
Conclusion
In this large cohort study, covering the period 1999–2019, patients were treated with individualized multimodal management. Mortality was not increased compared to the general population and comparable with recent registry studies from the Nordic countries and Europe. Overall cancer risk was slightly, but not significantly increased in the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a chronic disease caused by excessive secretion of growth hormone (GH) most often from a somatotroph pituitary adenoma, with subsequently increased levels of insulin-like growth factor 1 (IGF-1) [1]. Based on recent studies, the estimated prevalence is 28–137 cases/million inhabitants and the incidence varies between 2–11 cases/million per year [2,3,4,5], hence acromegaly is considered an orphan disease as defined by the European Union (European Medicines Agency, 02.09.2019). The clinical manifestations of acromegaly are caused by systemic effects related to the prolonged exposure to GH/IGF-1 excess (musculoskeletal, cardiovascular and metabolic comorbidities) and the local tumor extension (visual-field defects, cranial-nerve palsy and hypopituitarism) [1]. The metabolic complications, including insulin resistance and diabetes mellitus, increase the risk of cardiovascular-related morbidity and mortality [1, 6]. Further, increased levels of IGF-1 has been associated with increased risk for several malignancies [7], and studies have suggested GH and IGF-1 to facilitate a tumor microenvironment and neoplastic growth in the colon [8, 9].
Surgery is the only curative treatment. However, due to delayed diagnosis most patients with acromegaly present with macroadenomas (65–79%) and frequently invasive tumors [10]. Thus, the cure rate is disappointingly low when performed as primary treatment in clinical practice [11,12,13,14,15]. Treatment algorithms with focus on improving the surgical cure rate by preoperative medical treatment have been developed during the last two decades. The purpose of this individualized approach was to improve therapeutic outcomes and reduce the need for complicated and costly long-term medical therapy [10, 13, 15, 16]. The concept is based on relatively small prospective studies showing an improvement in surgical cure rate in newly diagnosed patients with acromegaly following somatostatin analogue (SSA) pretreatment, as compared to direct surgery [4, 17,18,19]. However, as the few RCT’s on the topic mostly provide short-time observation on the primary endpoint being cure rate, the concept is still debatable and well-planned studies with longer postoperative observation are warranted.
Before the millennium, mortality in patients with acromegaly was reported to be increased by two- to three- fold compared to the general population. However, since the millennium overall mortality rates have declined [2]. This change has been ascribed to the modern, multimodal therapy [2, 20]. According to a large meta-analysis, malignancies have become the leading cause of death [2]. Recent studies suggest that the type of cancers related to mortality in acromegaly are diverse, and not restricted to those traditionally associated with acromegaly, such as colorectal and thyroid cancer [2]. Thus, studies indicate that when mortality in acromegaly declines by modernized treatment, causes of death in acromegaly shift towards ageing and environmental factors, similar as in the general population [2].
Recent meta-analyses have shown an elevated risk of cancer in patients with acromegaly [21]. However, conflicting results regarding cancer risk in patients with acromegaly have been described, including population based studies from the United Kingdom and Germany that showed a lower cancer incidence in patients when compared to controls (standardized incidence ratio (SIR) 0.76 (95% confidence interval (CI) 0.60–0.95) and 0.75 (95% CI: 0.55–1.00), respectively) [22, 23].
The aim of the present single center cohort study was to investigate incidence, prevalence, overall mortality and the risk of cancer in a clinically well-defined cohort of patients with acromegaly with complete register-based follow-up.
Material and methods
Study design and population
Oslo University Hospital (OUS) is the tertiary referral center for patients with acromegaly in the South-Eastern Health Region of Norway, which is the regional health authority for about 3 million inhabitants, approximately 56% of the total Norwegian population (South-Eastern Norway Regional Health Authority, 16.11.2020). Between August 1999 and December 2019, 262 patients with newly diagnosed acromegaly were managed at OUS (Section of Specialized Endocrinology). This comprises patients included in the Preoperative Treatment of Acromegaly (POTA) study between 1999 and 2005 [4, 17, 18], and thereafter patients from our internal pituitary quality registry. The included patients underwent a standardized diagnostic work up, as established by the POTA protocol [4, 17, 18]. The patients were followed prospectively, and clinical, biochemical and radiological findings were recorded during a standardized set of serial visits, from diagnosis at baseline and following the treatment on a yearly basis. Patients with invasive and/or macroadenomas were usually offered primary SSA treatment for an individualized time period (in general minimum 6 months), before subsequent transsphenoidal surgery. Treatment was carefully tailored by a multidisciplinary approach according to the most recent recommendations [10, 12,13,14,15,16]. Treatment information during the time of follow-up including surgical procedures, medical treatment (1st generation SSAs, Pasireotide, dopamine agonists (DAs), GH receptor antagonists (GHRAs)) and radiotherapy was recorded consecutively during regular visits. During the study period, different assays for GH and IGF-1 were used, and we used morning GH levels (μg/L) for the statistical analysis as described previously [24]. IGF-1 is presented as the ratio of measured IGF-1 values, divided by the age-specific upper limit of normal (IGF-1/ULN).
A control cohort was obtained from the general population of the South-Eastern Health Region of Norway by the National Population Register and the Norwegian Tax Administration. The comparison cohort consisted of 100 age - and gender matched persons for every patient (n = 26,200). Date of diagnosis was considered the index date for the acromegaly patients and the matched controls. Demographics for the total population of the South-Eastern Health Region of Norway were obtained by The National Statistical Institute of Norway (National Statistical Institute of Norway, 04.11.2020).
Follow-up started at the date of diagnosis and the matched index date for the control cohort members. The follow-up period ended at the time of death or at the end of study (December 31, 2019).
Cause of death and cancer data
We received data regarding cause and date of death, and cancer diagnosis and localization from the Norwegian Cause of Death Registry (Norwegian Cause of Death Registry, 03.05.2022) and Cancer Registry of Norway (Cancer Registry of Norway, 02.05.2022). The cancer diagnoses were coded according to the International Classification of Diseases 10th Revision (ICD-10), and categorized into major cancer groups. Registry entries defined as benign by the Cancer registry of Norway (including pituitary adenomas) were excluded from the cancer analyses. International rules for multiple primary cancers (ICD-0 third edition) were used to define multiple primary neoplasms when reporting the data on cancer diagnoses and incidences [25]. For estimation of cancer incidence, only cancer diagnoses established after the index date were considered. However, all established cancer diagnoses between date of birth and end of follow-up were considered, when describing cancer events over time in relation to date of acromegaly diagnosis or the corresponding index date in the control cohort. Cancer events were adjusted for persons at risk per year.
Statistical analyses
We estimated the annual incidence rate of acromegaly per 106 persons-years based on the total population of South-Eastern Health Region of Norway for each calendar year, and the mean annual incidence rate. The point prevalence was estimated per million inhabitants in 2019, the last year of the study. Kaplan–Meier analysis was used to generate survival curves and Cox regression was used for time to event analysis. Using the comparison cohort as a reference, hazard ratios (HRs) with 95% CIs for mortality were estimated. Additionally, we divided the study period into three periods, and HRs for the periods 1999–2005, 2006–2012 and 2013–2019 were estimated, to analyze the potential change in mortality over time. To investigate cause-specific mortality, cause of death was categorized into main groups according to the leading causes of death observed in the acromegaly cohort; cardiovascular (any cardiovascular death), cancer (any cancer death) and other (any death that was not cardiovascular or cancer). Cox regression was used to investigate potential risk factors for mortality for predefined baseline characteristics (age, sex, tumor size, IGF-1/ULN values and first treatment modality). As only the first treatment was considered and this was close to baseline, immortal time bias was not considered an issue. We calculated and compared cancer incidence in the case and the control cohorts. Data are presented as median (interquartile range (IQR)) for continuous measures, and n (%) for categorical variables. For comparison of medians, Wilcoxon rank-sum test was used. Statistical analyses were executed by using STATA version 16.1.
Results
Patient characteristics
A total of 262 patients with acromegaly and 26,200 age- and sex-matched controls were included, 50.4% women. The mean follow-up time was 9.2 (SD: 6.0, range (0.0–20.4)) years. The median age at diagnosis was 48.0 years (37.6–58.0) and was constant over time. There was no major difference in median age at diagnosis between men and women (49.0 (37.8–59.2) and 47.7 (37.5–57.2), respectively (p = 0.700)). In the acromegaly cohort the median baseline GH and IGF-1/ULN were 7.9 (3.4–18.8) μg/L and 2.5 (1.8–3.4), respectively. At baseline, the median IGF-1/ULN was 2.4 (1.6–3.1) in women and 2.6 (1.9–3.5) in men. Radiological assessments were available for 229 patients, of whom 21% had microadenomas (<10 mm) and 79% had macroadenomas (≥10 mm). As a first treatment, 48% received surgery and 44% received 1st generation SSAs (remaining listed in Table 1). Six percent was treated with radiotherapy at some time point, and 8% underwent multiple surgeries. At the last visit, median GH had decreased to 1.1 (0.4–2.8) μg/L and median IGF-1/ULN to 0.9 (0.7–1.1). Of a total of 240 patients with available IGF-1/ULN values and with more than at least one year follow-up time, 62% were in remission (defined as IGF-1/ULN levels < 1). Medical treatment for acromegaly (including 1st generation SSAs, Pasireotide, DAs, GHRAs, or medical combination treatment) was received by 49% of patients, and 51% did not receive any treatment. Due to pituitary deficiency, 32% received hormone replacement therapy at the last visit (Table 1).
Incidence and prevalence
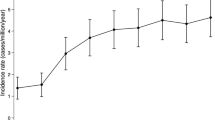
The point prevalence of acromegaly was 83 (95% CI: 72.6–93.5) cases/106 in 2019. The mean annual incidence rate was 4.7 (95% CI: 4.2–5.3) cases/106 persons, and remained constant over time.
Mortality
During the follow-up period, 14 patients (5%) with acromegaly died; 5 from cancer, 3 from cardiovascular disease and 6 from other causes (including multiple sclerosis, unspecified ileus, unspecified diabetes mellitus with renal complications, unexplained instantaneous death and missing). The overall mortality risk in acromegaly was not increased compared to the general population: HR 0.83 (95% CI: 0.49–1.41), p = 0.501 (Fig. 1).
For patients diagnosed between 1999–2005, 2006–2012 and 2013–2019 the HRs for death were 0.88 (95% CI: 0.42–1.85), 0.86 (95% CI: 0.39–1.92) and 0.52 (95% CI: 0.07–3.74), respectively (Fig. 2).
Cancer-specific and cardiovascular-specific mortality risks were not clearly increased in patients with acromegaly (HR 0.74 (95% CI: 0.31–1.79) and 0.80 (95% CI: 0.26–2.48), respectively). Age was the only factor at baseline associated with mortality risk in acromegaly (HR 1.16 (95% CI 1.07–1.26)), whereas sex, IGF-1/ULN levels, tumor size at diagnosis, and first treatment modality (surgery or SSAs) did not influence mortality risk (Table 2).
Cancer
Of the patients with acromegaly, 28 (10.7%) developed cancer following the diagnosis of acromegaly, as compared to 2063 (7.9%) of the matched controls after the corresponding index date. The HR for all cancers was 1.45 (95% CI: 1.0–2.1), p = 0.052. There were 3 cases of thyroid cancer (1.1%) in the acromegaly cohort and 19 (0.1%) in the control cohort. The HR for thyroid cancers was increased in patients with acromegaly as compared to the controls (HR: 17.0 (95% CI: 5.0–58.1), p < 0.001). We found no increased risk for other cancers, however, there was a borderline significant increased risk of prostate cancer in male patients with acromegaly (HR 2.05 (95% CI: 0.97–3.37), p = 0.060) (Table 3). Figure 3 illustrates all established cancer diagnoses in the study adjusted for persons at risk per year. There was a total of 48 cancer diagnoses in the acromegaly cohort, and 3211 in the control cohort, when including both cancers diagnosed before and after the diagnosis of acromegaly and index date, respectively. There was an increased rate of cancer diagnoses in the period around acromegaly diagnosis (±2 years) (Fig. 3).
Cancer incidence rate over time in patients with acromegaly and matched controls. Cancer diagnoses are presented per 100 person years in the control and acromegaly cohort distributed in relation to time of acromegaly diagnosis/index date (Year 0). The time categories cover two years each, e.g., “0” covers the time interval from time of diagnosis/index date until two years after diagnosis, and “−2” covers two years before diagnosis until time diagnosis/index date
Discussion
In the present prospective, single center cohort study from the South-Eastern Region of Norway, we found a constant incidence rate of acromegaly over time, and the mortality for patients diagnosed in the last two decades was persistently not elevated. There was a trend towards increased cancer incidence in patients with acromegaly. No acromegaly-related risk factors for death could be identified.
The prevalence and incidence of acromegaly in the present study are in line with previous estimates from the Nordic countries [4, 5, 26,27,28,29], indicating a good coverage of the patient population.
We did not demonstrate increased mortality in patients with acromegaly compared to the general population, in contrast to the slightly elevated rates in Sweden and Denmark [3, 26, 30, 31]. Possible explanations may be that the present analyses are based on a more recent cohort (1999–2019) than the analyses from Sweden (1987–2013 [3, 31] and 1991–2011 [30]) and Denmark (1991–2010 [26]). This is supported by the decline in mortality in Sweden in the patients diagnosed more recently [31], and is coinciding with the broader availability of effective medication for acromegaly and improved surgical and radiation techniques, enabling multi-modal, individualized treatment to patients with acromegaly. Although the overall mortality in the most recent Swedish publication was increased, the mortality in patients with biochemical control was not elevated, in contrast to non-controlled patients [30]. Due to few events, no firm conclusion on the most recently diagnosed patients could be drawn, as indicated by a broad CI in our study. However, similar trends with improved survival have been demonstrated previously in meta-analyses [2, 20].
In the present study, we could not identify any baseline characteristics with significant influence on mortality, except for age, as expected. In order to avoid immortal time bias, only baseline characteristics were included in our analysis [32]. The majority of our patients had pituitary macroadenomas, and almost half of them received SSAs as a primary treatment, because the probability for surgical cure was considered to be low in many of these cases. This practice was regularly implemented in the patients diagnosed in the early 2000s, when the first randomized study on preoperative SSA treatment was initiated [4]. This change in treatment, together with the modernized and individualized approach over the last twenty years, had an important effect on mortality. When mortality in acromegaly decreases towards the background population, causes of patient death shift towards causes in the general population [2]. Thus, the leading cause of death in the Norwegian population at present is malignancies, with cardiovascular disease as the second, in accordance with our findings in the patients with acromegaly (Norwegian Cause of Death Registry, 10.06.2021).
We observed an equal gender distribution and median age at diagnosis, in contrast to a meta-analysis demonstrating a moderate female predominance (53%), and a higher age at diagnosis in women as compared to men [33]. In comparison, a recent review found that acromegaly was more prevalent in women than in men, and women were older at diagnosis [34]. However, in the Nordics, the gender distribution was equal, and age at diagnosis for men and women was similar [26,27,28, 33, 34]. Studies derived from national registries, like the Danish and Swedish studies and the present study, are representative for a large, unselected populations, and are thus little prone to selection bias.
Cancer incidence increased with age in the control cohort as expected, whereas, in the acromegaly cohort cancer incidence peaked markedly around the time of acromegaly (Fig. 3). This could be ascribed to surveillance bias occurring after a cancer diagnosis resulting in the detection of acromegaly, or vice versa: Newly diagnosed acromegaly may prompt cancer screening or suspicion. In order to reduce surveillance bias when estimating cancer incidence and risk, we excluded cases of malignancy established before the diagnosis of acromegaly, in accordance with the recent Swedish population-based study [35]. In contrast to the registry-based cohort study from Denmark [21], we did not exclude cancers established one year after the acromegaly diagnosis in order to avoid discharging cancers that could be associated with acromegaly, and possibly reflect biologic effects of GH excess in the years before delayed acromegaly diagnosis. Similarly to our results, the referred studies from Scandinavia found an overall elevated cancer risk in patients with acromegaly, and the meta-analysis in the Danish publication added further support to these findings [21, 35]. Accordingly, a nationwide cohort study from Italy demonstrated an overall increased cancer risk in patients compared to the general population [36]. However, this is in contrast with the population based studies from the United Kingdom and Germany, where no increased cancer incidence in patients with acromegaly compared to the general population were demonstrated [22, 23].
Of all five thyroid cancer cases in our patient cohort, two were established before the diagnosis of acromegaly and three after. Only two of these five cases where diagnosed close to the diagnosis (+/− 2 years) of acromegaly. However, we cannot exclude that the elevated thyroid cancer risk in our study can be related to surveillance bias. As described, data on thyroid cancer in patients with acromegaly are controversial and the absolute numbers are low [21, 35, 36]. Interestingly, we found a borderline significant increased risk of prostate cancer in men with acromegaly. These findings are similar to the above mentioned Danish meta-analysis [21], but in contrast to the studies from the United Kingdom and Germany [22, 23]. Previous studies have shown that increased IGF-1 levels were associated with increased risk of prostate cancer [7]. For cancer categories with low incidence, no finite conclusions can be drawn, as there is a considerable risk for type 2 error.
The strengths of this study is that it is a single center study with patients followed according to a standardized management course and long follow-up interval for of up to 20 years, in combination with data from national health-registries, and the comparison with a large matched cohort based on the general population. Despite the single center design, the study cohort is population based representing unselected cases from over 3 million inhabitants. Although the study covers a large geographical area and population followed up to two decades, the absolute numbers of death and cancers were low.
Conclusion
The incidence, prevalence, gender distribution and age at diagnosis of acromegaly in South-Eastern Norway is similar to data from the other Nordic countries. The mortality rates in the patient cohort, that has received modern multimodal therapies and active surveillance for acromegaly-related complications, was not different from the background population. As in other recent European studies, we found a trend towards an increased overall cancer risk. Cardiovascular- and cancer-related mortality were not different as compared to the general population.
References
S. Melmed, M.D. Bronstein, P. Chanson, A. Klibanski, F.F. Casanueva, J.A.H. Wass et al. A Consensus Statement on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 14(9), 552–61. (2018)
F. Bolfi, A.F. Neves, C.L. Boguszewski, V.S. Nunes-Nogueira, Mortality in acromegaly decreased in the last decade: a systematic review and meta-analysis. Eur. J. Endocrinol. 179(1), 59–71 (2018)
D. Esposito, O. Ragnarsson, D. Granfeldt, T. Marlow, G. Johannsson, D.S. Olsson, Decreasing mortality and changes in treatment patterns in patients with acromegaly from a nationwide study. Eur. J. Endocrinol. 178(5), 459–69. (2018)
S.M. Carlsen, M. Lund-Johansen, T. Schreiner, S. Aanderud, O. Johannesen, J. Svartberg et al. Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J. Clin. Endocrinol. Metab. 93(8), 2984–2990 (2008)
C. Aagaard, A.S. Christophersen, S. Finnerup, C. Rosendal, H.A. Gulisano, K.S. Ettrup, et al. The prevalence of acromegaly is higher than previously reported: changes over a three-decade period. Clin. Endocrinol. 97(6), 773–782 (2022)
D. Esposito, D.S. Olsson, S. Franzén, M. Miftaraj, J. Nåtman, S. Gudbjörnsdottir et al. Effect of diabetes on morbidity and mortality in patients with acromegaly. J. Clin. Endocrinol. Metab. 107(9), 2483–92. (2022)
A.G. Renehan, M. Zwahlen, C. Minder, S.T. O’Dwyer, S.M. Shalet, M. Egger, Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363(9418), 1346–1353 (2004)
V. Chesnokova, S. Zonis, C. Zhou, M.V. Recouvreux, A. Ben-Shlomo, T. Araki et al. Growth hormone is permissive for neoplastic colon growth. Proc. Natl Acad. Sci. USA 113(23), E3250–E3259 (2016)
L. Kasuki, B. Maia, M.R. Gadelha, Acromegaly and colorectal neoplasm: an update. Front Endocrinol. 13, 924952 (2022)
J. Bollerslev, A. Heck, N.C. Olarescu, Management of endocrine disease: individualised management of acromegaly. Eur. J. Endocrinol. 181(2), R57–r71 (2019)
M. Bex, R. Abs, G. T’Sjoen, J. Mockel, B. Velkeniers, K. Muermans et al. AcroBel–the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur. J. Endocrinol. 157(4), 399–409 (2007)
J. Bollerslev, S.L. Fougner, J.P. Berg, New directions in pharmacological treatment of acromegaly. Expert Opin. Investigational drugs 18(1), 13–22 (2009)
M. Fleseriu, A.R. Hoffman, L. Katznelson, American Association of Clinical Endocrinologists and American College of Endocrinology Disease State clinical review: management of acromegaly patients: what is the role of pre-operative medical therapy? Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinologists 21(6), 668–673 (2015)
A. Giustina, P. Chanson, M.D. Bronstein, A. Klibanski, S. Lamberts, F.F. Casanueva et al. A consensus on criteria for cure of acromegaly. J. Clin. Endocrinol. Metab. 95(7), 3141–3148 (2010)
L. Katznelson, E.R. Laws Jr., S. Melmed, M.E. Molitch, M.H. Murad, A. Utz et al. Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(11), 3933–3951 (2014)
M. Losa, J. Bollerslev, Pros and cons in endocrine practice: pre-surgical treatment with somatostatin analogues in acromegaly. Endocrine 52(3), 451–457 (2016)
S.M. Carlsen, J. Svartberg, T. Schreiner, S. Aanderud, O. Johannesen, S. Skeie et al. Six-month preoperative octreotide treatment in unselected, de novo patients with acromegaly: effect on biochemistry, tumour volume, and postoperative cure. Clin. Endocrinol. 74(6), 736–743 (2011)
S.L. Fougner, J. Bollerslev, J. Svartberg, M. Oksnes, J. Cooper, S.M. Carlsen, Preoperative octreotide treatment of acromegaly: long-term results of a randomised controlled trial. Eur. J. Endocrinol. 171(2), 229–235 (2014)
V.S. Nunes, J.M. Correa, M.E. Puga, E.M. Silva, C.L. Boguszewski, Preoperative somatostatin analogues versus direct transsphenoidal surgery for newly-diagnosed acromegaly patients: a systematic review and meta-analysis using the GRADE system. Pituitary 18(4), 500–508 (2015)
O.M. Dekkers, N.R. Biermasz, A.M. Pereira, J.A. Romijn, J.P. Vandenbroucke, Mortality in acromegaly: a metaanalysis. J. Clin. Endocrinol. Metab. 93(1), 61–67 (2008)
J. Dal, M.Z. Leisner, K. Hermansen, D.K. Farkas, M. Bengtsen, C. Kistorp et al. Cancer incidence in patients with acromegaly: a cohort study and meta-analysis of the literature. J. Clin. Endocrinol. Metab. 103(6), 2182–2188 (2018)
S.M. Orme, R.J. McNally, R.A. Cartwright, P.E. Belchetz, Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J. Clin. Endocrinol. Metab. 83(8), 2730–2734 (1998)
D. Petroff, A. Tönjes, M. Grussendorf, M. Droste, C. Dimopoulou, G. Stalla et al. The incidence of cancer among acromegaly patients: results from the German Acromegaly Registry. J. Clin. Endocrinol. Metab. 100(10), 3894–3902 (2015)
N.C. Olarescu, A. Heck, K. Godang, T. Ueland, J. Bollerslev, The metabolic risk in patients newly diagnosed with acromegaly is related to fat distribution and circulating adipokines and improves after treatment. Neuroendocrinology 103(3-4), 197–206 (2016)
Working Group Report. International rules for multiple primary cancers (ICD-0 third edition). Eur. J. Cancer Prev. 14(4), 307–308 (2016)
J. Dal, U. Feldt-Rasmussen, M. Andersen, L.O. Kristensen, P. Laurberg, L. Pedersen et al. Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur. J. Endocrinol. 175(3), 181–190 (2016)
A. Tjornstrand, K. Gunnarsson, M. Evert, E. Holmberg, O. Ragnarsson, T. Rosen et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur. J. Endocrinol. 171(4), 519–526 (2014)
T.T. Agustsson, T. Baldvinsdottir, J.G. Jonasson, E. Olafsdottir, V. Steinthorsdottir, G. Sigurdsson et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur. J. Endocrinol. 173(5), 655–664 (2015)
R. Kauppinen-Mäkelin, T. Sane, A. Reunanen, M.J. Välimäki, L. Niskanen, H. Markkanen et al. A nationwide survey of mortality in acromegaly. J. Clin. Endocrinol. Metab. 90(7), 4081–4086 (2005)
S. Arnardóttir, J. Järås, P. Burman, K. Berinder, P. Dahlqvist, E.M. Erfurth et al. Long-term outcomes of patients with acromegaly: a report from the Swedish Pituitary Register. Eur. J. Endocrinol. 186(3), 329–39. (2022)
D. Esposito, O. Ragnarsson, D. Granfeldt, T. Marlow, G. Johannsson, D.S. Olsson, Decreasing mortality and changes in treatment patterns in patients with acromegaly from a nationwide study. Eur. J. Endocrinol. 180(2), X1–X3 (2019)
O.M. Dekkers, R.H.H. Groenwold, When observational studies can give wrong answers: the potential of immortal time bias. Eur. J. Endocrinol. 184(1), E1–e4 (2021)
J. Dal, B.G. Skov, M. Andersen, U. Feldt-Rasmussen, C.L. Feltoft, J. Karmisholt et al. Sex differences in acromegaly at diagnosis: a nationwide cohort study and meta-analysis of the literature. Clin. Endocrinol. 94(4), 625–35. (2021)
N.F. Lenders, A.I. McCormack, K.K.Y. Ho, Management of endocrine disease: does gender matter in the management of acromegaly? Eur. J. Endocrinol. 182(5), R67–r82 (2020)
D. Esposito, O. Ragnarsson, G. Johannsson, D.S. Olsson, Incidence of benign and malignant tumors in patients with acromegaly is increased: a nationwide population-based study. J. Clin. Endocrinol. Metabol. 106(12), 3487–3496 (2021)
M. Terzolo, G. Reimondo, P. Berchialla, E. Ferrante, E. Malchiodi, L. De Marinis et al. Acromegaly is associated with increased cancer risk: a survey in Italy. Endocr. Relat. Cancer 24(9), 495–504 (2017)
Acknowledgements
C.M.F. received a scholarship provided by Southern and Eastern Norway Regional Health Authority (Helse Sør-Øst). Data on cause of death were obtained from the Norwegian Cause of Death Registry. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Author contributions
All authors contributed to the formulation of the scientific question and study design. All authors participated in material acquisition and preparation. C.M.F. and O.M.D. performed the statistical analyses. All authors contributed to the data interpretation. C.M.F. and A.H. wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and accepted the submission of the final manuscript.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.H. has received speaker fees from Recordati and Ipsen. J.B. has received speaker fees from Ipsen and Pfizer, and has served as an advisory board member for Pfizer. N.C.O. received speaker fees from CORE2ED (supported by a medical education grant from Ipsen). O.M.D. and C.M.F. have nothing to declare.
Ethics approval
The study was approved by the regional ethics committee (REK no: 15240) and the hospital authority.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falch, C.M., Olarescu, N.C., Bollerslev, J. et al. Trends in incidence and mortality risk for acromegaly in Norway: a cohort study. Endocrine 80, 152–159 (2023). https://doi.org/10.1007/s12020-022-03275-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03275-6