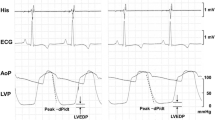
Abstract
Tyrosine kinase inhibitors are known to clinically induce various types of cardiovascular adverse events; however, it is still difficult to predict them at preclinical stage. In order to explore how to better predict such drug-induced cardiovascular adverse events, we tried to develop a new protocol by assessing acute electrophysiological, cardiohemodynamic, and cytotoxic effects of dasatinib in vivo and in vitro. Dasatinib at 0.03 and 0.3 mg/kg was intravenously administered to the halothane-anesthetized dogs for 10 min with an interval of 20 min between the dosing (n = 4). Meanwhile, that at 0.1, 0.3, and 1 μM was cumulatively applied to the human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) (n = 7). In the dogs, the low and high doses provided peak plasma concentrations of 40 ± 5 (0.08) and 615 ± 38 ng/mL (1.26 μM), respectively. The low dose decreased the heart rate, impaired the left ventricular mechanical function, and prolonged the ventricular effective refractory period. The high dose prolonged the repolarization period, induced hemorrhagic tendency, and increased plasma cardiac troponin I level in addition to enhancement of the changes observed after the low dose, whereas it neither affected the cardiac conduction nor induced ventricular arrhythmias. In the hiPSC-CMs, dasatinib prolonged the repolarization and refractory periods like in dogs, while it did not induce apoptotic or necrotic process, but that it increased the conduction speed. Clinically observed major cardiovascular adverse events of dasatinib were observed qualitatively by currently proposed assay protocol, which may become a useful guide for predicting the cardiotoxicity of new tyrosine kinase inhibitors.








Similar content being viewed by others
References
Druker, B. J. (2008). Translation of the Philadelphia chromosome into therapy for CML. Blood,112, 4808–4817.
Leber, B. (2011). CML biology for the clinician in 2011: six impossible things to believe before breakfast on the way to cure. Current Oncology,18, e185–e190.
Hochhaus, A., O’Brien, S. G., Guilhot, F., Druker, B. J., Branford, S., Foroni, L., et al. (2009). Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia,23, 1054–1061.
Shah, N. P., Guilhot, F., Cortes, J. E., Schiffer, C. A., le Coutre, P., Brümmendorf, T. H., et al. (2014). Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood,123, 2317–2324.
Force, T., Krause, D. S., & Van Etten, R. A. (2007). Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Reviews Cancer,7, 332–344. (Review).
Moslehi, J. J., & Deininger, M. (2015). Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. Journal of Clinical Oncology,33, 4210–4218. (Review).
Bristol-Myers Squibb Company. (2017). Sprycel (dasatinib) prescribing information. Princeton, NJ: Bristol-Myers Squibb Compan. Retrieved February 11, 2019 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021986s020lbl.pdf.
Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., et al. (2015). The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell,14, 644–658.
Negulescu, A. M., & Mehlen, P. (2018). Dependence receptors: The dark side awakens. The FEBS Journal,285, 3909–3924. (Review).
Justice, J. N., Nambiar, A.M., Tchkonia, T., LeBrasseur, N. K., Pascual, R., Hashmi, S. K., et al. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, in press.
Cortes, J. E., Saglio, G., Kantarjian, H. M., Baccarani, M., Mayer, J., Boqué, C., et al. (2016). Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. Journal of Clinical Oncology,34, 2333–2340.
Brave, M., Goodman, V., Kaminskas, E., Farrell, A., Timmer, W., Pope, S., et al. (2008). Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clinical Cancer Research,14, 352–359.
Zhang, D. Y., Wang, Y., Lau, C. P., Tse, H. F., & Li, G. R. (2008). Both EGFR kinase and Src-related tyrosine kinases regulate human ether-à-go-go-related gene potassium channels. Cellular Signalling,20, 1815–1821.
Lu, Z., Wu, C. Y., Jiang, Y. P., Ballou, L. M., Clausen, C., Cohen, I. S., et al. (2012). Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Science Translational Medicine,4, 131ra50.
Nagaraj, C., Tang, B., Bálint, Z., Wygrecka, M., Hrzenjak, A., Kwapiszewska, G., et al. (2013). Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. European Respiratory Journal,41, 85–95.
Phan, C., Jutant, E. M., Tu, L., Thuillet, R., Seferian, A., Montani, D., et al. (2018). Dasatinib increases endothelial permeability leading to pleural effusion. European Respiratory Journal,51, 1701096.
Sugiyama, A., & Hashimoto, K. (1998). Effects of gastrointestinal prokinetic agents, TKS159 and cisapride, on the in situ canine heart assessed by cardiohemodynamic and electrophysiological monitoring. Toxicology and Applied Pharmacology,152, 261–269.
Sugiyama, A. (2008). Sensitive and reliable proarrhythmia in vivo animal models for predicting drug-induced torsades de pointes in patients with remodelled hearts. British Journal of Pharmacology,154, 1528–1537.
Yamamoto, W., Asakura, K., Ando, H., Taniguchi, T., Ojima, A., Uda, T., et al. (2016). Electrophysiological characteristics of human iPSC-derived cardiomyocytes for the assessment of drug-induced proarrhythmic potential. PLoS ONE,11, e0167348.
Ando, H., Yoshinaga, T., Yamamoto, W., Asakura, K., Uda, T., Taniguchi, T., et al. (2017). A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. Journal of Pharmacological and Toxicological Methods,84, 111–127.
Izumi-Nakaseko, H., Nakamura, Y., Wada, T., Ando, K., Kanda, Y., Sekino, Y., et al. (2017). Characterization of human iPS cell-derived cardiomyocyte sheets as a model to detect drug-induced conduction disturbance. Journal of Toxicological Sciences,42, 183–192.
Izumi-Nakaseko, H., Hagiwara-Nagasawa, M., Naito, A. T., Goto, A., Chiba, K., Sekino, Y., et al. (2018). Application of human induced pluripotent stem cell-derived cardiomyocytes sheets with microelectrode array system to estimate antiarrhythmic properties of multi-ion channel blockers. Journal of Pharmacological Sciences,137, 372–378.
Wada, T., Ando, K., Naito, A. T., Nakamura, Y., Goto, A., Chiba, K., et al. (2018). Sunitinib does not acutely alter left ventricular systolic function, but induces diastolic dysfunction. Cancer Chemotherapy and Pharmacology,82, 65–75.
Wu, A. H. B. (2017). Release of cardiac troponin from healthy and damaged myocardium. Frontiers in Laboratory Medicine,1, 144–150.
Dohi, T., Maehara, A., Brener, S. J., Généreux, P., Gershlick, A. H., Mehran, R., et al. (2015). Utility of peak creatine kinase-MB measurements in predicting myocardial infarct size, left ventricular dysfunction, and outcome after first anterior wall acute myocardial infarction (from the INFUSE-AMI trial). American Journal of Cardiology,115, 563–570.
Van de Van de, A., Verheyen, J., Xhonneux, R., & Reneman, R. S. (1989). An improved method to correct the QT interval of the electrocardiogram for changes in heart rate. Journal of Pharmacological and Toxicological Methods,22, 207–217.
Nakamura, Y., Matsuo, J., Miyamoto, N., Ojima, A., Ando, K., Kanda, Y., et al. (2014). Assessment of testing methods for drug-induced repolarization delay and arrhythmias in an iPS cell-derived cardiomyocyte sheet: Multi-site validation study. Journal of Pharmacological Sciences,124, 494–501.
Sugiyama, A., Takahara, A., & Hashimoto, K. (1999). Electrophysiologic, cardiohemodynamic and beta-blocking actions of a new ultra-short-acting beta-blocker, ONO-1101, assessed by the in vivo canine model in comparison with esmolol. Journal of Cardiovascular Pharmacology,34, 70–77.
Izumi-Nakaseko, H., Kanda, Y., Nakamura, Y., Hagiwara-Nagasawa, M., Wada, T., Ando, K., et al. (2017). Development of correction formula for field potential duration of human induced pluripotent stem cell-derived cardiomyocytes sheets. Journal of Pharmacological Sciences,135, 44–50.
DrugBank Version 5.1.2. Retrieved February 18, 2019 from https://www.drugbank.ca/drugs/DB01254(dasatinib).
de Campaigno, E. P., Bondon-Guitton, E., Laurent, G., Montastruc, F., Montastruc, J. L., Lapeyre-Mestre, M., et al. (2017). Identification of cellular targets involved in cardiac failure caused by PKI in oncology: an approach combining pharmacovigilance and pharmacodynamics. British Journal of Clinical Pharmacology,83, 1544–1555.
Mingard, C., Paech, F., Bouitbir, J., & Krähenbühl, S. (2018). Mechanisms of toxicity associated with six tyrosine kinase inhibitors in human hepatocyte cell lines. Journal of Applied Toxicology,38, 418–431.
Will, Y., Dykens, J. A., Nadanaciva, S., Hirakawa, B., Jamieson, J., Marroquin, L. D., et al. (2008). Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicological Sciences,106, 153–161.
Sovari, A. A., Iravanian, S., Dolmatova, E., Jiao, Z., Liu, H., Zandieh, S., et al. (2011). Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. Journal of the American College of Cardiology,58, 2332–2339.
Doherty, K. R., Wappel, R. L., Talbert, D. R., Trusk, P. B., Moran, D. M., Kramer, J. W., et al. (2013). Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicology and Applied Pharmacology,272, 245–255.
Sharma, A., Burridge, P. W., McKeithan, W. L., Serrano, R., Shukla, P., Sayed, N., et al. (2017). High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science Translational Medicine,9, eaaf2584.
Matsuo, J., Nakamura, Y., Izumi-Nakaseko, H., Ando, K., Sekino, Y., & Sugiyama, A. (2015). Possible effects of inhibition of IKr and IKs on field-potential waveforms in the human iPS cell-derived cardiomyocytes sheet. Journal of Pharmacological Sciences,128, 92–95.
Yang, B., & Papoian, T. (2018). Preclinical approaches to assess potential kinase inhibitor-induced cardiac toxicity: Past, present and future. Journal of Applied Toxicology,38, 790–800.
Quintás-Cardama, A., Kantarjian, H., Ravandi, F., O’Brien, S., Thomas, D., Vidal-Senmache, G., et al. (2009). Bleeding diathesis in patients with chronic myelogenous leukemia receiving dasatinib therapy. Cancer,115, 2482–2490.
Astedt, B. (1987). Clinical pharmacology of tranexamic acid. Scandinavian Journal of Gastroenterology. Supplement,137, 22–25.
Nash, C. A., Séverin, S., Dawood, B. B., Makris, M., Mumford, A., Wilde, J., et al. (2010). Src family kinases are essential for primary aggregation by Gi-coupled receptors. Journal of Thrombosis and Haemostasis,8, 2273–2282.
Li, R., Grosser, T., & Diamond, S. L. (2017). Microfluidic whole blood testing of platelet response to pharmacological agents. Platelets,28, 457–462.
Cortes, J., Mauro, M., Steegmann, J. L., Saglio, G., Malhotra, R., Ukropec, J. A., et al. (2015). Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: Data from the FDA adverse event reporting system. American Journal of Hematology,90, E66–E72.
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M., & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology,160, 1577–1579.
McGrath, J., Drummond, G., McLachlan, E., Kilkenny, C., & Wainwright, C. (2010). Guidelines for reporting experiments involving animals: The ARRIVE guidelines. British Journal of Pharmacology,160, 1573–1576.
Acknowledgements
The authors thank Dr. Yuji Nakamura for the conduct of in vivo experiments and the data analysis, Mrs. Yuri Ichikawa for technical assistance, Mr. Kentaro Tanaka and Miss Hikaru Tsuruoka for in vitro data analysis, and Alpha MED Scientific, Inc. for technical advice.
Funding
This work was supported by JSPS KAKENHI Grant Number 17K08608 (to HI-N), 15K08246 (to KA), and 16K08559 (to AS); AMED Grant 18mk0104117j0001 (to YK); AMED Grant 18am0101122j0002 (to ATN and AS); Toho University’s 60th anniversary (to KA); and Initiative for Realizing Diversity in the Research Environment (to HI-N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicated no potential conflicts of interest for this study project.
Ethical Approval
All experiments were approved by the Animal Research Committee for Animal Experimentation of Toho University (No. 17-52-324) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Toho University and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for reporting experiments involving animals [44, 45].
Additional information
Handling Editor: Bérengère Dumotier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Izumi-Nakaseko, H., Fujiyoshi, M., Hagiwara-Nagasawa, M. et al. Dasatinib can Impair Left Ventricular Mechanical Function But May Lack Proarrhythmic Effect: A Proposal of Non-clinical Guidance for Predicting Clinical Cardiovascular Adverse Events of Tyrosine Kinase Inhibitors. Cardiovasc Toxicol 20, 58–70 (2020). https://doi.org/10.1007/s12012-019-09538-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09538-5