Abstract
Background
Bariatric surgery is the most effective long-term treatment of severe obesity. Unfortunately, many patients experience inadequate weight loss, weight plateau, or weight recidivism. We sought to determine the efficacy of high-dose liraglutide (3.0 mg once daily) in patients with prior bariatric surgery.
Methods
We performed a retrospective chart review of 33 consecutive patients, aged 18–65, who received liraglutide for weight loss in the setting of any previous bariatric surgery. Indications were weight recidivism (> 10% weight regain from lowest post-surgical weight), inadequate weight loss (< 20% weight loss from initial clinic assessment, or pre-surgical weight if unavailable), and plateau (patient desires further weight loss but does not fit into either other category). Our primary outcomes were median percentage weight loss and median BMI change at 16 and 28 weeks, inclusive of time taken to titrate the medication to target dose. Secondary outcomes were the presence of adverse effects and the need to discontinue the medication.
Results
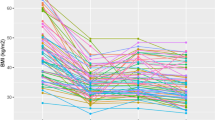
Of a total of 33 patients identified, 20 met inclusion criteria and had adequate data to be included in our analysis. At 16 weeks median percentage weight loss was 7.1% (IQR 5.1–12.2%), and at 28 weeks 9.7% (IQR 7.8–13.9%). Median BMI change was 3.5 kg/m2 (16 weeks, IQR 2.2–4.6 kg/m2) and 4.7 kg/m2 (28 weeks, IQR 3.7−5.6 kg/m2). There were no major adverse events.
Conclusions
High-dose liraglutide is an effective adjunct treatment for weight loss in patients with prior bariatric surgery.


Similar content being viewed by others
References
Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Sjostrom CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–84.
De Gara CJ, Karmali S. The anatomy of a weight recidivism and revision bariatric surgical clinic. Gastroenterol Res Pract. 2014;2014:721095.
Karmali S, Brar B, Shi X, et al. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23(11):1922–33.
Sheppard CE, Lester ELW, Chuck AW, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. 2017;13(3):491–500. Impact of Weight Regain. Gastroenterology Research and Practice. 2013;2013:379564
Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37(11):1443–51.
Novo Nordisk Canada Inc. Saxenda® Product Monograph. [Internet]. 2017 [cited 2018 Feb 5]. Available from: http://www.novonordisk.ca/content/dam/Canada/AFFILIATE/www-novonordisk-ca/OurProducts/PDF/Saxenda_PM_English.pdf.
European Medicines Agency. Guideline on Clinical Evaluation of Medicinal Products used in Weight Control. [Internet]. 2014 [cited 2018 Feb 5]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/07/WC500170278.pdf.
Pajecki D, Halpern A, Cercato C, et al. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir. 2013;40(3):191–5.
Creange C, Lin E, Ren-Fielding C, et al. Use of liraglutide for weight loss in patients with prior bariatric surgery. Surg Obes Relat Dis. 2016;12(7):S157.
Gorgojo-martínez JJ, Feo-ortega G, Serrano-moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis. 2016;12(10):1856–63.
Stanford FC, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Surg Obes Relat Dis. 2017;13(3):491–500.
Lau D et al. Early weight loss responders to liraglutide 3.0 mg had greater weight loss, regression to normoglycemia and reduced T2D development at 3 years vs. early non-responders: SCALE obesity and prediabetes. Can J Diabetes. 2016;40(5):S33.
Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–99.
Pi-sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015;35(10):926–34.
Laferrère B. Bariatric surgery and obesity: influence on the incretins. Int J Obes Suppl. 2016;6(Suppl 1):S32–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author 1: The author declares no conflict of interest.
Author 2: Novo Nordisk Canada (travel and speaker fees), Shire Pharma Canada (development of educational materials, speaker fees), and Valeant Canada (travel and speaker fees).
Author 3: Novo Nordisk Canada (travel, speaker, and consultant fees), and Valeant (consultant fees).
Author 4: Novo Nordisk (travel, speaker, consultant fees, and advisory board), Merck (travel, speaker, and consultant fees), and Valeant (advisory board).
Ethics and Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Rye, P., Modi, R., Cawsey, S. et al. Efficacy of High-Dose Liraglutide as an Adjunct for Weight Loss in Patients with Prior Bariatric Surgery. OBES SURG 28, 3553–3558 (2018). https://doi.org/10.1007/s11695-018-3393-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3393-7