Abstract
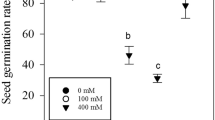
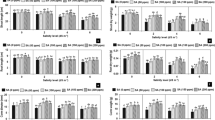
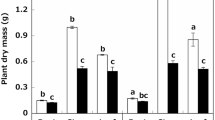
The effect of salinity on some morpho-physiological characteristics in lisianthus cultivars was investigated. Cultivars namely, Blue Picotee (C1), Champagne (C2), Lime Green (C3), and Pure White (C4), were subjected to salt stress (0–60 mM NaCl) in a sand culture and their responses were measured. Our results showed that as a salinity level increased, growth parameters, relative water content, photosynthetic pigments, and gas-exchange characteristics decreased in all cultivars, while root fresh mass, root/shoot length ratio, electrolyte leakage, and a malondialdehyde content increased. However, the changes were less pronounced in C3 and C4 compared to C1 and C2. The regression analysis of the relationship between salinity levels and seedling height or root/shoot length ratio defined two groups with different slope coefficients: C1 and C2 as salt-sensitive cultivars and C3 and C4 as salt-tolerant cultivars. Shoot dry mass and leaf area tolerance indices were less affected by salinity in C3 and C4 compared to those in C1 and C2. Further, C3 and C4 showed higher photosynthetic rates, greater stomatal conductances, and accumulated greater K+ and Ca2+ contents and K+/Na+ ratios in roots and shoots compared to those in C1 and C2. The results suggests that C3 and C4 could be recommended as resistant cultivars due to maintaining higher growth, water balance, leaf gas exchange, ion compartmentalization, and lower lipid peroxidation in response to salinity compared to C1 and C2.
Similar content being viewed by others
Abbreviations
- C1 :
-
Blue Picotee
- C2 :
-
Champagne
- C3 :
-
Lime Green
- C4 :
-
Pure White
- Chl:
-
chlorophyll
- DM:
-
dry mass
- EL:
-
electrolyte leakage
- E :
-
transpiration rate
- FM:
-
fresh mass
- g s :
-
stomatal conductance
- LA:
-
leaf area
- MDA:
-
malondialdehyde
- P N :
-
net photosynthetic rate
- RW C:
-
relative water content
- TI:
-
tolerance index
References
Akram M. S., Ashraf M., Akram N. A.: Effectiveness of potassium sulfate in mitigating salt-induced adverse effects on different physio-biochemical attributes in sunflower (Helianthus annuus L.). — Flora 204: 471–483, 2009.
Anonymous: Standard Methods for the Examination of Water and Wastewater, Vol. 2. Pp. 4–132. American Public Health Association, American Water Works Association, Water Pollution Control Federation, & Water Environment Federation., Washington D.C. 1915.
Bongi G., Palliotti A.: Olive. — In: Shaffer B., Anderson P.C. (ed.): Handbook of Environmental Physiology of Fruit Crops. Temperate Crops, Vol. 1. Pp.165–187. CRC Press, Boca Raton 1994.
Cassaniti C., Leonardi C., Flowers T.J.: The effects of sodium chloride on ornamental shrubs. — Sci. Hortic.-Amsterdam 122: 586–593, 2009.
Chartzoulakis K.S.: Salinity and olive: growth, salt tolerance, photosynthesis and yield. — Agr. Water Manage. 78: 108–121, 2005.
Chartzoulakis K., Loupassaki M., Bertaki M. et al.: Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. — Sci. Hortic.-Amsterdam 96: 235–247, 2002.
DeRidder B.P., Salvucci M.E.: Modulation of Rubisco activase gene expression during heat stress in cotton (Gossypium hirsutum L.) involves post-transcriptional mechanisms. — Plant Sci. 172: 246–254, 2007.
Dionisio-Sese M.L., Tobita S.: Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. — J. Plant Physiol. 157: 54–58, 2000.
Essa T.: Effect of salinity stress on growth and nutrient composition of three soybean (Glycine max L. Merrill) cultivars. — J. Agron. Crop Sci. 188: 86–93, 2002.
Farooq S., Azam F.: The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. — J. Plant Physiol. 163: 629–637, 2006.
Flowers T., Yeo A.: Breeding for salinity resistance in crop plants: where next? — Funct. Plant Biol. 22: 875–884, 1995.
Gómez-Pérez L., Valdez-Aguilar L.A., Sandoval-Rangel A. et al.: Calcium ameliorates the tolerance of lisianthus [Eustoma grandiflorum (Raf.) Shinn.] to alkalinity in irrigation water. — HortScience 49: 807–811, 2014.
Gunes A., Inal A., Alpaslan M. et al.: Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. — J. Plant Physiol. 164: 728–736, 2007.
Hafsi C., Lakhdhar A., Rabhi M. et al.: Interactive effects of salinity and potassium availability on growth, water status, and ionic composition of Hordeum maritimum. — J. Plant Nutr. Soil Sc. 170: 469–473, 2007.
Izadi M. H., Rabbani J., Emam Y. et al.: Effects of salinity stress on physiological performance of various wheat and barley cultivars. — J. Plant Nutr. 37: 520–531, 2014.
Jia W., Wang Y., Zhang S. et al.: Saltstressinduced ABA accumulation is more sensitively triggered in roots than in shoots. — J. Exp. Bot. 53: 2201–2206, 2002.
Katsuhara M., Otsuka T., Ezaki B.: Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. — Plant Sci. 169: 369–373, 2005.
Lichtenthaler H.K.: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. — Methods Enzymol. 148: 350–382, 1987.
Lutts S., Kinet J., Bouharmont J.: NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. — Ann. Bot.-London 78: 389–398, 1996.
Maggio A., Raimondi G., Martino A. et al.: Salt stress response in tomato beyond the salinity tolerance threshold. — Environ. Exp. Bot. 59: 276–282, 2007.
Maksimović I., Putnik-Delić M., Gani I. et al.: Growth, ion composition, and stomatal conductance of peas exposed to salinity. — Open Life Sciences. 5: 682–691, 2010.
Mavrogianopoulos G., Savvas D., Vogli V.: Influence of NaClsalinity imposed on half of the root system of hydroponically grown tomato on growth, yield, and tissue mineral composition. — Environ. Exp. Bot. 77: 557–564, 2002.
Meloni D.A., Oliva M.A., Martinez C.A. et al.: Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. — Environ. Exp. Bot. 49: 69–76, 2003.
Mills H.A., Jones J.B., Wolf B.: Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide. Pp. 422. MicroMacro Publishing, Athens 1996.
Morales M., Sánchez-Blanco M., Olmos E. et al.: Changes in the growth, leaf water relations and cell ultrastructure in Argyranthemum coronopifolium plants under saline conditions. — J. Plant Physiol. 153: 174–180, 1998.
Munns R.: Genes and salt tolerance: bringing them together. — New Phytol. 167: 645–663, 2005.
Navarro A., Bañón S., Conejero W. et al.: Ornamental characters, ion accumulation and water status in Arbutus unedo seedlings irrigated with saline water and subsequent relief and transplanting. — Environ. Exp. Bot. 62: 364–370, 2008.
Pattanagul W., Thitisaksakul M.: Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. — Indian J. Exp. Biol. 46: 736–742, 2008.
Pérez-Tornero O., Tallón C.I., Porras I. et al.: Physiological and growth changes in micropropagated Citrus macrophylla explants due to salinity. — J. Plant Physiol. 166: 1923–1933, 2009.
Poustini K., Siosemardeh A.: Ion distribution in wheat cultivars in response to salinity stress. — Field Crop. Res. 85: 125–133, 2004.
Rengasamy P.: World salinization with emphasis on Australia. — J. Exp. Bot. 57: 1017–1023, 2006.
Ruiz-Carrasco K., Antognoni F., Coulibaly A.K. et al.: Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. — Plant Physiol. Bioch. 49: 1333–1341, 2011.
Saab I.N., Sharp R.E., Pritchard J. et al.: Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. — Plant Physiol. 93: 1329–1336, 1990.
Sairam R.K., Rao K.V., Srivastava G.: Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. — Plant Sci. 163: 1037–1046, 2002.
Shahbaz M., Ashraf M., Akram N.A. et al.: Salt-induced modulation in growth, photosynthetic capacity, proline content and ion accumulation in sunflower (Helianthus annuus L.). — Acta Physiol. Plant. 33: 1113–1122, 2011.
Sharma L., Kaushal M., Bali S. K. et al.: Evaluation of rough lemon (Citrus jambhiri Lush.) as rootstock for salinity tolerance at seedling stage under in vitro conditions. — Afr. J. Biotechnol. 12: 6267, 2013.
Shi G., Cai Q.: Cadmium tolerance and accumulation in eight potential energy crops. — Biotechnol. Adv. 27: 555–561, 2009.
Shimizu-Yumoto H., Ichimura K.: Combination pulse treatment of 1-naphthaleneacetic acid and aminoethoxyvinylglycine greatly improves postharvest life in cut Eustoma flowers. — Postharvest Biol. Tec. 56: 104–107, 2010.
Tabatabaei S., Ehsanzadeh P.: Photosynthetic pigments, ionic and antioxidative behaviour of hulled tetraploid wheat in response to NaCl. — Photosynthetica. 54: 340–350, 2016.
Tarakcioglu C., Inal A.: Changes induced by salinity, demarcating specific ion ratio (Na/Cl) and osmolality in ion and proline accumulation, nitrate reductase activity, and growth performance of lettuce. — J. Plant Nutr. 25: 27–41, 2002.
Tarchoune I., Degl’Innocenti E., Kaddour R. et al.: Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. — Acta Physiol. Plant. 34: 607–615, 2012.
Turhan A., Şeniz V.: Salt tolerance of some tomato genotypes grown in Turkey. — J. Food Agric. Environ. 8: 332–339, 2010.
Valdez-Aguilar L. A., Grieve C.M., Poss J.A.: Response of Lisianthus to irrigation with saline water: plant growth. — J. Plant Nutr. 36: 1605–1614, 2013.
Valdez-Aguilar L.A., Grieve C.M., Poss J.A.: Response of Lisianthus to irrigation with saline water: ion relations. — J. Plant Nutr. 37: 546–561, 2014.
Walsh L.M., Douglas L.A.: Instrumental Methods for Analysis of Soils and Plant Tissue. Pp. 500. Soil Science Society of America, Madison 1972
Wang F., Zeng B., Sun Z. et al.: Relationship between proline and Hg2+-induced oxidative stress in a tolerant rice mutant. — Arch. Environ. Con. Tox. 56: 723–731, 2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashrafi, N., Rezaei Nejad, A. Lisianthus response to salinity stress. Photosynthetica 56, 487–494 (2018). https://doi.org/10.1007/s11099-017-0709-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-017-0709-0