Abstract
Purpose
Three- dimensional (3D) printing has received significant attention as a manufacturing process for pharmaceutical dosage forms. In this study, we used Fusion Deposition Modelling (FDM) in order to print “candy – like” formulations by imitating Starmix® sweets to prepare paediatric medicines with enhanced palatability.
Methods
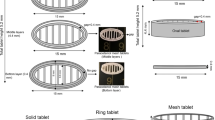
Hot melt extrusion processing (HME) was coupled with FDM to prepare extruded filaments of indomethacin (IND), hypromellose acetate succinate (HPMCAS) and polyethylene glycol (PEG) formulations and subsequently feed them in the 3D printer. The shapes of the Starmix® objects were printed in the form of a heart, ring, bottle, ring, bear and lion. Differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), Fourier Transform Infra-red Spectroscopy (FT-IR) and confocal Raman analysis were used to assess the drug – excipient interactions and the content uniformity.
Results
Physicochemical analysis showed the presence of molecularly dispersed IND in the printed tablets. In vivo taste masking evaluation demonstrated excellent masking of the drug bitterness. The printed forms were evaluated for drug dissolution and showed immediate IND release independently of the printed shape, within 60 min.
Conclusions
3D printing was used successfully to process drug loaded filaments for the development of paediatric printed tablets in the form of Starmix® designs.










Similar content being viewed by others
Abbreviations
- DSC:
-
Differential scanning calorimetry
- FT-IR:
-
Fourier Transform Infra-red Spectroscopy
- HME:
-
Hot melt extrusion processing
- HPLC:
-
High performance liquid chromatography
- HPMCAS:
-
Hypromellose acetate succinate
- IND:
-
Indomethacin
- PEG:
-
Polyethylene glycol
- SEM:
-
Scanning Electron Microscopy
- TGA:
-
Thermogravimetric analysis
- XRPD:
-
X-ray powder diffraction
References
Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs—a review of commercially available oral formulations. J Pharm Sci. 2008;97:1731–74.
Formulations of choice for the paediatric population. European Medicines Agancy (2006). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf.
Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin Drug Deliv. 2015;12:1727–40.
Benavides S, Huynh D, Morgan J, Briars L. Approach to the pediatric prescription in a community pharmacy. J Pediatr Pharmacol Ther. 2011;16(4):298–307.
Yin HS, Mendelsohn AL, Wolf MS, Parker RM, Fierman A, van Schaick L, et al. Parents' medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164:181–6.
Mennella JA, Roberts KM, Mathew PS, Reed DR. Children’s perceptions about medicines: individual differences and taste. BMC Pediatr. 2015;15:130.
Turner MA, Catapano M, Hirschfeld S, Giaquinto C. Paediatric drug development: The impact of evolving regulations. Adv Drug Deliv Rev. 2014;73:2–13.
Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm Res. 2016;33(8):1817–32.
Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50.
Sandler N, Preis M. Printed drug-delivery systems for improved patient treatment. Trends Pharmacol Sci. 2016;37(12):1070–80.
Preis M, Öblom H. 3D-printed drugs for children-Are we ready yet? AAPS Pharm SciTech. 2017;18(2):303–8.
Sachs E, Cima M, Cornie J. Three-dimensional printing: rapid tooling and prototypes directly from a CAD model. CIRP Ann Manuf, Techn. 1990;39:201–4.
Khaled SA, Burley JC, Alexander MR, Roberts CR. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461:105–11.
Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496:414–20.
Water JJ, Bohr A, Boetker J, Aho J, Sandler N, Nielsen HM, et al. Three-dimensional printing of drug-eluting implants: Preparation of an antimicrobial polylactide feedstock material. J Pharm Sci. 2015;104:1099–107.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–8.
Busch SF, Weidenbach M, Fey M, Schäfer F, Probst T, Koch M. Optical properties of 3D printable plastics in the THz regime and their application for 3D printed THz optics. J Infrared Millim Terahertz Waves. 2014;35:993–7.
Goyanes A, Buanz A, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–62.
Goyanes A, Martinez PR, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharma. 2015;494:657–63.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7.
Okwuosa TC, Stefaniak D, Arafat B, Isreb A, Wan K-W, Alhnan MA. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm Res. 2016;33(11):2704–12.
Maniruzzaman M, Boateng JB, Bonnefille M, Aranyos A, Mitchell JC, Douroumis D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80:433–42.
Goyanes A, Buanz AB, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharma. 2014;476:88–92.
Genina N, Holländer J, Jukarainen H, Mäkilä E, Salonen J, Sandler N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2015;30(90):53–63.
Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modelling. Int J Pharm. 2016;509:255–63.
Boetker J, Water JJ, Aho J, Arnfast L, Bohr A, Rantanen J. Modifying release characteristics from 3D printed drug-eluting products. Eur J Pharm Sci. 2016;90:47–52.
El-Badry M, Fetih G, Fathy M. Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm J. 2009;17:217–25.
Zhang G-C, Lin H-L, Lin S-Y. Thermal analysis and FTIR spectral curve-fitting investigation of formation mechanism and stability of indomethacin-saccharin cocrystals via solid-state grinding process. J Pharm Biomed Anal. 2012;66:162–9.
Lim RTY, Ng WK, Tan RB. Dissolution enhancement of indomethacin via amorphization using co-milling and supercritical co-precipitation processing. Powder Tech. 2013;240:79–87.
Bandi N, Wei W, Roberts CB, Kotra LP, Kompella UB. Preparation of budesonide–and indomethacin–hydroxypropyl-β-cyclodextrin (HPBCD) complexes using a single-step, organic-solvent-free supercritical fluid process. Eur J Pharm Sci. 2004;23:159–68.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and Indomethacin in Amorphous molecular dispersions. Pharm Res. 1997;14:1691–8.
Ewing AV, Clarke GS, Kazarian SG. Stability of indomethacin with relevance to the release from amorphous solid dispersions studied with ATR-FTIR spectroscopic imaging. Eur J Pharm Sci. 2014;60:64–71.
Chauhan H, Kuldipkumar A, Barder T, Medek A, Gu C-H, Atef E. correlation of inhibitory effects of polymers on Indomethacin precipitation in solution and amorphous solid crystallization based on molecular interaction. Pharm Res. 2014;31:500–15.
Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, Turner R, et al. E.F. Initiative, Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients. Adv Drug Deliv Rev. 2014;73:14–33.
Maniruzzaman M, Boateng JS, Chowdhry BZ, Snowden MJ, Douroumis D. A review on the taste masking of bitter APIs: hot-melt extrusion (HME) evaluation. Drug Dev Ind Pharm. 2014;40:145–56.
Gryczke A, Schminke S, Maniruzzaman M, Beck J, Douroumis D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf B: Biointerfaces. 2011;86:275–84.
Rachid Q, Rawas-Qalaji M, Estelle F, Simons R, Simons KJ. Dissolution testing of sublingual tablets: A novel In vitro method. AAPS PharmSciTech. 2011;12(2):544–52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scoutaris, N., Ross, S.A. & Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm Res 35, 34 (2018). https://doi.org/10.1007/s11095-017-2284-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2284-2