Abstract
The stiffness of the cardiovascular environment changes during ageing and in disease and contributes to disease incidence and progression. For instance, increased arterial stiffness can lead to atherosclerosis, while stiffening of the heart due to fibrosis can increase the chances of heart failure. Cells can sense the stiffness of the extracellular matrix through integrin adhesions and other mechanosensitive structures and in response to this initiate mechanosignalling pathways that ultimately change the cellular behaviour. Over the past decades, interest in mechanobiology has steadily increased and with this also our understanding of the molecular basis of mechanosensing and transduction. However, much of our knowledge about the mechanisms is derived from studies investigating focal adhesions in non-muscle cells, which are distinct in several regards from the cell–matrix adhesions in cardiomyocytes (costameres) or vascular smooth muscle cells (dense plaques or podosomes). Therefore, we will look here first at the evidence for mechanical sensing in the cardiovascular system, before comparing the different cytoskeletal arrangements and adhesion sites in cardiomyocytes and vascular smooth muscle cells and what is known about mechanical sensing through the various structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction (i.e. why care about mechanosensing in the cardiovascular system?)
Cardiovascular diseases (CVD), including coronary artery disease, myocardial infarctions (MI), hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) are the primary cause of mortality worldwide and a huge economic burden. CVD costs the US both, directly and indirectly, $320 billion per year, with this figure set to rise to $918 billion by 2030 (Travers et al. 2016). Similarly, in the EU, CVD costs €169 billion per year, equating to 62% of their healthcare costs. This is contributed to by deaths as well as the long-term impact of a reduced quality of life and life expectancies (Leal et al. 2006). Clinically, all these conditions have in common a stiffening of the extracellular matrix (ECM) through fibrosis and it has become clear that this stiffening is a key contributing factor in the progression of the CVD. The cells in the heart or vessel walls sense the changes to chemical and mechanical signalling and respond to the restructuring of the ECM by changing their phenotype; e.g. cardiomyocytes become less contractile due to changes in gene expression and myofibrillar organisation (Ward and Iskratsch 2019), while vascular smooth muscle cells can switch from a contractile to a synthetic phenotype that allows them to start migrating or invading into the inner layer of the vessel wall (Hartman et al. 2016; McDaniel et al. 2007).
Our knowledge about mechanosensing is mostly derived from migratory cells e.g. in cancer, while information about mechanosensing through specific cardiomyocyte or vascular smooth muscle adhesions is lacking. A PubMed keyword search for “mechanotransduction” and “Focal Adhesion” for instance yields 467 papers, while the combination with “Costamere” or with “Podosome” comes up with only ten results each. Of course, this keyword search is not showing the complete picture. Nonetheless, it demonstrates a certain focus of the mechanobiology field on motile cells e.g. in cancer where focal adhesions are the primary mechanosensitive structures. Here we want to disseminate the similarities and differences between the different integrin adhesion structures. For this, we will first look at the changes to the ECM composition and stiffness in the heart and the vessel wall respectively, before discussing the different cell–matrix adhesions in cardiac and VSMCs and their involvement in mechanical sensing.
Structure of the cardiac ECM and mechanical changes in heart disease
In the heart, the ECM provides physical support and facilitates cellular functions (Chang et al. 2016). It helps to connect and organise the myocytes, absorb and transmit forces generated on the tissue and thereby prevent damage during systole and diastole (Weber et al. 1994). Cardiac fibroblasts produce the majority of the cardiac ECM. The cardiac collagen fibrils are mainly comprised of collagens type I and III. Collagen type IV sits in the basement membrane which surrounds the myocytes (Brown et al. 2005; Lockhart et al. 2011; Rienks et al. 2014). Other constituents of the ECM include glycoproteins (e.g. fibronectin and laminin), proteoglycans (especially heparan sulfate proteoglycans) and elastin. These molecules interact with cell surface receptors, such as integrins which link the ECM to the internal cytoskeletal network and function also as mechanical sensing and signalling hubs (Awada et al. 2016; Baudino et al. 2006; Ward and Iskratsch 2019). However, the composition of the ECM is dynamic and changes during development and in disease. Fibronectin and collagen are important binding partners in the developing heart, but laminin is up-regulated, becoming the main integrin binding partner in the adult heart (Marijianowski et al. 1994; Oliviero et al. 2000; Terracio et al. 1991).
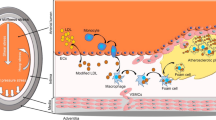
Diseases or injuries, such as MI damage the myocardium, with ischaemia triggering an inflammatory response, changing the molecular composition and cellular morphology. The abnormal ECM constituents change the functionality of the heart (Awada et al. 2016; Sullivan et al. 2014). After an MI, the death of cardiomyocytes en masse triggers a cascade resulting in fibrotic tissue replacing the dead cells. Inflammation activates immune pathways and oxidative stress, leading to the mobilisation of toll-like receptors which activates NF-κB, resulting in a further inflammatory pathway upregulation. The inflammatory cytokines (TNF-α, IL-1β and IL-6) cause cellular apoptosis and fibrotic tissue formation (Bujak et al. 2009). The effects of NF-κB can be protective, however, prolonged presence induces a damaging inflammatory response. Inflammatory cytokines attract lymphocytes and macrophages to the site, removing dead cardiomyocytes. Matrix Metalloproteinases (MMPs) are activated, degrading the ECM, causing the accumulation of collagen particles (Etoh et al. 2001). This activates neutrophils, further enhancing the inflammatory response. MMPs, ROS and the acidic environment of damaged tissue activate TGF-β1 release, which stimulates inflammatory cells, enhancing fibrotic remodelling (Kong et al. 2014). The remodelling itself includes the activation of the CFs, secretion of the stiffer collagen I versus III, as well as fibronectin and importantly also lysyl oxidases that lead to increased cross-linking of the ECM (Hermida et al. 2009; Hughes et al. 2008; Maki 2009; Marijianowski et al. 1994; McCormick et al. 1994; McCormick and Thomas 1998). Overall, these changes result in an increased stiffness of the fibrotic myocardium, from approx. 10 kPa in the healthy to > 50 kPa in the infarcted or fibrotic tissue although there is currently still a large variability depending on the model system and techniques used to measure the stiffness (Ward and Iskratsch 2019).
Structure of the vessel wall ECM and disease associated mechanical changes
Changes to the vessel structure, associated with ageing and atherosclerosis are dramatically increasing the risk of CVD. These structural changes lead to altered topography as well as enhanced stiffness (Gozna et al. 1973; Lakatta et al. 1987; Laurent et al. 2001). Because of this, macroscale analysis of vascular stiffness, such as pulse wave velocity is used to predict the risk for cardiovascular disease. Arteries are composite structures (Wagenseil and Mecham 2009). The intimal layer contains basement membrane and endothelial cells. It is separated from the media by an internal elastic lamina, containing longitudinal elastin fibres. The medial layer is the largest contributor to the elastic properties of the arterial wall. It is populated by smooth muscle cells and contains collagen and circumferential elastin fibres. The outer adventitia layer contains fibroblasts and a looser collagen and elastin matrix. Owing to increased association of VSMCs with collagen under pressure, arteries display non-linear stress–strain patterns (i.e. the elastic modulus increases when they are stretched through blood flow) (Bank et al. 1996; Wagenseil and Mecham 2009; Zhou and Fung 1997). The measured moduli differ between the various arteries and e.g. pulmonary arteries are stiffer than the aorta and further the descending thoracic aortic tissue is stiffer than ascending aortic tissue (de Beaufort et al. 2018; Grant and Twigg 2013). The measurements further vary between longitudinal and circumferential strain. Moreover, Young’s moduli are sometimes separately measured for elastic lamellae, elastin-collagen fibers and collagen fibers (Khamdaeng et al. 2012). Finally, the mechanical properties differ between macro and microscale (Kohn et al. 2015). At the microscale, elastic moduli between 20 and 70 kPa have been measured with nanoindentation or micropipette aspiration assays (Hanna et al. 2014; Hemmasizadeh et al. 2012; Matsumoto and Hayashi 1996), however, no large-scale study of changes during ageing have been performed. At the macroscale (circumferential and longitudinal) moduli from 100 kPa to several MPa have been measured with a strong trend from low stiffness in childhood to high values in old age and diseases, including atherosclerosis or in patients with aneurisms (Khanafer et al. 2011). These changes are largely coming from fragmentation, calcification and degradation of elastin fibres, shifting the load increasingly onto stiffer collagen fibrils (Kohn et al. 2015; Schlatmann and Becker 1977). Additionally, collagen expression and crosslinking by non-enzymatic glycation increases with age, further stiffening the vessel walls (Schlatmann and Becker 1977; Schleicher et al. 1997; Sims et al. 1996).
Evidence for rigidity sensing of cardiac and smooth muscle cells
It is well established that many cell types, including cardiomyocytes and vascular smooth muscle cells, can sense the changes in the ECM stiffness, through changes in the transmission of mechanical forces, altering their behavioural response accordingly (Iskratsch et al. 2014; Roca-Cusachs et al. 2012). Cell area, contraction frequency, percentage of contractile (isolated) cells, force and contractile work of primary or stem cell derived cardiomyocytes change in response to matrix stiffness (Engler et al. 2008; Hazeltine et al. 2012; Hersch et al. 2013; Li et al. 2016; Pandey et al. 2018; Ribeiro et al. 2015; Rodriguez et al. 2011, 2014). Generally the studies found that contractile force increases with stiffness (Hazeltine et al. 2012; Hersch et al. 2013; Rodriguez et al. 2011), however when micropatterning was used to restrict the cell area and shape there was a peak in contractile force at 10 kPa, while at higher stiffnesses myofibrillar rupture led to reduced mechanical forces (Ribeiro et al. 2015). Also, contractile work was found to be highest at 10 kPa corresponding to the native tissue stiffness (Engler et al. 2008). From a signalling pathway perspective cardiomyocytes respond to the stiffness by changing expression of cardiac developmental agonists such as PI3K/AKT or p38/JNK pathway components, or antagonists (e.g. canonical Wnt signalling components), whereby differences exist between cells that are exposed to a static stiffness or a hydrogel that becomes stiffer over time (i.e. mimicking the changing stiffness during heart development)(Young et al. 2014). Also lipid pathways (Li et al. 2016) calcium handling (Rodriguez et al. 2011; van Deel et al. 2017) and possibly also nitric oxide signalling (Jian et al. 2014) are affected by the stiffness and presumably involved in the mechanosignalling.
Vascular smooth muscle cells similarly respond to a range of physical/mechanical cues including pressure, stretch, topography and stiffness (Chaterji et al. 2014; Kim et al. 2015; Le et al. 2018b; McDaniel et al. 2007). Matrix stiffness (and stiffness gradient) can induce a phenotypic switch of VSMC and affects VSMC stress fibre organisation, contractility or migration rate (Isenberg et al. 2009; Peyton et al. 2008; Peyton and Putnam 2005; Sugita et al. 2019), although effects in 3D were less pronounced than in 2D (Peyton et al. 2008). Stiffness further regulated VSMC cell cycle and proliferation (Bae et al. 2014; Klein et al. 2009). Furthermore, VSMC can form podosomes at soft 3D matrices without chemical stimuli (Furmaniak-Kazmierczak et al. 2007). Podosomes are protrusive structures at the matrix interface of the plasma membrane. They are a critical element of the phenotypic switch from a contractile to a synthetic phenotype (Hartman et al. 2016; McDaniel et al. 2007) and regulate matrix adhesion and matrix degradation through the targeted secretion of matrix metalloproteinases (MMPs) (Vijayakumar et al. 2015).
Mechanical sensing through cell–matrix adhesions: common themes and differences between cell types
A comparison of proteomic studies of the adhesion complexes from various cell types resulted in a 2412-protein integrin adhesome (Horton et al. 2015). However only 60 of these were present in at least five data sets, indicating a large variability between cell types and conditions. Approximately half of the consensus adhesome was matching a literature curated adhesome, while the rest was previously less well characterized (Winograd-Katz et al. 2014).
This suggests that although there are important differences in the composition (as well as the organisation of the attached cytoskeleton and the force transmission from the cytoskeleton), key proteins are shared between different cell types and presumably also adhesion structures in cardiomyocytes and smooth muscle cells; indeed many focal adhesion proteins have been initially isolated from smooth muscle such as chicken gizzard (Burridge and Connell 1983; Feramisco and Burridge 1980; Geiger 1979; Kelly et al. 1987).
In all cell-ECM structures integrins connect to the ECM and span through the membrane. At the cytoplasmic tails are a range of integrin binding proteins, including kindlin, paxillin, or FAK, as well as actin binding proteins (e.g. vinculin or VASP), or proteins that bind both integrin and actin (e.g. talin, α-actinin, filamin) (Winograd-Katz et al. 2014). These complexes connect the integrins to the cytoskeleton and play signalling roles. Integrins are activated either outside-in through binding of an ECM ligand, or inside-out through binding of kindlin and talin that stabilize the open conformation and promote ECM ligand binding, while Filamin on the other hand inhibits integrin activity (Liu et al. 2015). Talin binds to integrins through its head domain and has two actin binding sites on the rod domain (and also a less-well characterized actin binding site on the head) which connect then to the actin cytoskeleton (Klapholz and Brown 2017; Legate and Fassler 2009). If force is applied through the cytoskeleton (either through myosin contractions or actin assembly) this can lead to stretching of talin and opening of cryptic vinculin binding sites on stiff environments, while the force will be transmitted through the entire complex onto the matrix and deform the matrix on soft environments (Roca-Cusachs et al. 2012). Vinculin then reinforces the connection by binding to both talin and actin. Nevertheless, talin’s connection to actin is not continuous but rather it cyclically engages and disengages in what has been termed a stick–slip mechanism, leading to repeated extension of talin (Margadant et al. 2011). Similarly, even though some integrins can form catch bonds and therefore integrin-ECM bond lifetimes increase under force (Roca-Cusachs et al. 2012), they are still only in the order of seconds (Kong et al. 2009), thus making focal adhesions dynamic structures. Due to the repeated engagement and disengagement the cell-ECM adhesion is frequently compared to a molecular clutch (Elosegui-Artola et al. 2018; Mitchison and Kirschner 1988; Swaminathan and Waterman 2016). An updated model of the molecular adhesion clutch suggests a load and fail cycle wherein the force initially builds up resulting in talin stretching and recruitment of vinculin and adhesion reinforcement. Eventually the force build-up leads however to bond destabilisation, and clutch disengagement as well as release of all forces in a catastrophic event. On soft substrates, the rate of load transmission is below the integrin–ECM bond lifetime and the bond dissociates before talin can unfold. Above the optimal rigidity, force builds up too fast for additional clutches to stabilize the adhesion and therefore it disengages (Elosegui-Artola et al. 2016, 2018; Swaminathan and Waterman 2016). Integrins are structurally focal adhesions are elongated and consist of multiple subunits that are about 300 nm in diameter, each attached to a single stress fibre (Hu et al. 2015). The components are stratified into multiple layers that have been termed ‘integrin signalling’ (containing e.g. integrin cytoplasmic tails and talin head domains), ‘force transduction’ (containing e.g. talin rod domain and vinculin) and ‘actin regulatory’ layer (containing e.g. VASP) (Kanchanawong et al. 2010).
What are the differences?
But as demonstrated by the comparison of the proteomic analysis data, focal adhesions differ between cell types and even within a single cell depending on space and time (Horton et al. 2015). During adhesion formation early integrin clusters are connected to small contractile units (composed of non-muscle isoforms of muscle sarcomeric proteins, such as actin, myosin, α-actinin, tropomyosin, tropomodulin, the formin FHOD1, etc.) that apply force onto integrins and are involved in rigidity sensing (Iskratsch et al. 2013; Meacci et al. 2016; Wolfenson et al. 2016). In contrast, (mature) peripheral adhesions are connected to non-contractile dorsal stress fibres while central adhesions are attached to contractile ventral stress fibres (Tojkander et al. 2012). Contractile force to the peripheral adhesions is indirectly provided through transverse arcs that connect to the dorsal stress fibres, but is not sufficient for adhesion growth and mechanosensing, which additionally depends on the force provided through ARP2/3 dependent actin polymerization (Oakes et al. 2012, 2018; Pasapera et al. 2010). However tension on talin is higher in peripheral versus central adhesions, further suggesting that these are differently organised (Kumar et al. 2016).
To complicate matters further, even peripheral focal adhesions are not all the same and can be divided into (mechanically) stable or tugging focal adhesions, whereby the latter are exposed to fluctuations in the level of force and the position within the adhesion where the force is applied (Plotnikov et al. 2012); only the latter is required for mechanical sensing and e.g. durotaxis, i.e. the migration guided by a stiffness gradient.
Further, the different cell types vary in their organisation widely and this includes also different cytoskeletal organisation and cell–matrix interaction sites through which the cells can sense the mechanical stimuli (Figs. 1 & 2). Because mechanical sensing is depending both on the organisation of the (contractile force-producing) cytoskeleton as well as the cell–matrix adhesion sites, we next look at the different cytoskeletal arrangements and adhesion sites in cardiomyocytes and vascular smooth muscle cells and then compare what is known about the regulation of mechanical sensing.
The cardiomyocyte costamere. The sarcomeres are connected to the ECM through integrins, the dystrophin-glycoprotein complex (DGC) and desmin. a Side view of cardiomyocyte with thin filaments in red and thick filaments in light blue as well as Z-discs in grey and costamer in purple. b Side view of a costamere with the adhesion complex (i.e. talin, vinculin and other adaptor as well as signalling molecules) in purple, the DGC in orange and the desmin intermediate filaments in lime green. Arrows indicate the contractile forces from myofibrillar and non-myofibrillar structures. nmM non-muscle myosin. (Color figure online)
Vascular smooth muscle cell adhesions. a Top view of a VSCM, with dense bodies in grey, dense plaques in purple and podosomes in green. b Side view of dense plaques attached to contractile stress fibres and dense bodies in the cytoplasm. Arrows indicate contractile forces of the stress fibres. c Side view of a podosome. The forces of the protrusive core with branched actin, as well as the tensile force from the adhesion ring are indicated by arrows. (Color figure online)
Costameres
Costameres, the main matrix attachment sites in cardiomyocytes connect the cytoskeleton to the ECM not only through integrins and associated proteins, but also through the dystrophin-glycoprotein complex (DGC) (Fig. 1). Additionally, the costameres connect to the myofibrils through the intermediate filament protein desmin, whereby all three components appear to be involved in mechanical sensing and signal transduction (Ward and Iskratsch 2019). The integrin adhesion component has many of the proteins that are also found in focal adhesions, including talin and vinculin which attach to cytoplasmic γ-actin that is further connected to the sarcomeric Z-disc through actin crosslinkers such as α-actinin and plectin (Ervasti 2003). The attachment of the sarcomeres to the costameres through cytoplasmic actin leads to a situation where the forces from the regular sarcomeric contractions can be modified through non-muscle myosin, which contracts the cytoplasmic actin (Fig. 1) (Pandey et al. 2018). Moreover, non-muscle myosin is localized at the costameres especially in heart disease, suggesting that this modulation can lead to an alteration of mechanical sensing with potentially adverse effects on the disease progression (Pandey et al. 2018). Together the forces are sensed at the adhesions where it leads to different dynamics of talin stretching, depending on the stiffness of the ECM. Because talin has a large range of binding partners and all the rod domains can unfold and refold under force, such differences in stretching dynamics are expected to alter mechanical signal transduction beyond vinculin binding and adhesion reinforcement and indeed force dependent talin binding has been reported already for other proteins than vinculin (Haining et al. 2018; Klapholz and Brown 2017; Yao et al. 2016). In addition to talin the costameres contain a number of proteins that are general mechanosensors and included in the consensus adhesome (e.g. ILK-PINCH-Parvin) (Jani and Schock 2009; Li et al. 2012) as well as muscle specific proteins such as MLP (Flick and Konieczny 2000; Knoll et al. 2002). Importantly also, the isoforms of integrins and several adapter proteins are different in cardiomyocytes compared to many non-muscle cells (β1D vs β1A integrin, talin 2 vs talin 1) which affects binding affinities, dynamics and signaling (Hawkes et al. 2019; Ward and Iskratsch 2019). E.g. a reduced binding of kindlin and paxillin to β1D was reported, suggesting that talin binding might be the main activator of β1D integrin in muscle (Soto-Ribeiro et al. 2019; Yates et al. 2012). Moreover many isoforms switch back to embryonic splice variants in cardiac disease and thereby again modifying affinities and potentially other binding partners (Ward and Iskratsch 2019).
The intermediate filament protein desmin is flexible and seems to serve a function as load bearing spring, i.e. to absorb contractile forces between Z-disc, microtubules and ECM (Hein et al. 2000; Robison et al. 2016). Abnormal desmin levels and/or filament organisation are linked to heart disease presumably due to the lack of this force buffering capability (Bouvet et al. 2016; Clemen et al. 2015; Geisler and Weber 1988; Thornell et al. 1997).
The dystrophin glycoprotein complex (DGC) seems to serve a similar function as shock absorber (Le et al. 2018a). It consists of dystrophin, the transmembrane dystroglycan and sarcoglycan-sarcospan subcomplexes as well as the subsarcolemmal proteins dystrobrevins and syntrophins. Dystrophin binds to actin through its N-terminal and rod domain and to dystroglycan through the cysteine-rich C-terminal domain, while dystroglycan connects to laminin in the basement membrane (Lapidos et al. 2004). In the heart dystrophin is detected all along the membrane, albeit more concentrated at the costamere (Kawada et al. 2003; Stevenson et al. 1997). Dystrophin has roughly equal affinities to sarcomeric α-actin, as well as β- and γ-actin (Renley et al. 1998). However, it appears from immuno-electronmicrographs that it is mostly oriented near-parallel to the cytoplasmic surface, with only a few dystrophin molecules connecting to the farthest out myofibrillar actin filaments (Wakayama et al. 1993). Thus, it is assumed that it primarily interacts with subsarcolemmal γ-actin (Fig. 1) and through this interaction helps to protect sarcolemma from fragmentation or micro-rupturing and mitochondria from deformation during the myofibrillar contraction (Kawada et al. 2003). Therefore, absence of dystrophin, or other members of the DGC can lead to a progression of the dystrophic phenotype in hearts during aging, which is accelerated through physical exercise (Nakamura et al. 2002). Apart from this mechanical role the DGC is also a signalling hub and involved in ion transport regulation, nitric oxide, MAPK or Rac1 GTPase signalling [reviewed in (Constantin 2014)]. Therefore, some of these signalling pathways might be also regulated through the changing mechanical environments, or lack of buffering of the force in absence of the DGC.
Dense plaques
Historically dense plaques were defined by their appearance as densely stained structures in electron micrographs of smooth muscle cells (North et al. 1993). Not to be confused with cadherin-based cell–cell adherens junctions (Dorland and Huveneers 2017), or intermediate filament-based “dense bodies” (Tang 2008), dense plaques here are integrin-rich adhesion sites of smooth muscle cells with associated actin networks (Morgan et al. 2007; Turner et al. 1991; Zhang and Gunst 2008), and are responsible for force transmission among smooth muscle cells across the ECM (Gunst and Tang 2000) (Fig. 2a, b). Dense plaques again contain the same repertoire of molecules that are also present in focal adhesions, including integrins, α-actinin, talin, vinculin or filamin (many of which in fact were first identified in smooth muscle as discussed above) (Draeger et al. 1990; Turner et al. 1991), but additionally there are also components that are specific for smooth muscle, including the LIM domain protein LPP or metavinculin (which is also present at the costamere) (Bays et al. 2017; Van Itallie et al. 2014). Dystrophin, while present in smooth muscle is distinctly absent from the dense plaques, but rather localized to caveolae (North et al. 1993). Again there are also structural differences and dense plaques appear in cross sections as discrete foci at the membrane, however in longitudinal sections these structures can extend the entire length of the cell (Small 1985). Because dense plaques are directly coupled to contractile stress-fibres they might be comparable to central rather than peripheral focal adhesions. However, since the dense plaque adhesome has not been analysed yet to our knowledge, it is unclear how much they resemble each other. It is clear however that several key proteins at the dense plaques are regulated in response to forces in similar way as in non-muscle cells. e.g. Force development in smooth muscle cells has been proven to evoke talin and paxillin phosphorylation (Pavalko et al. 1995; Wang et al. 1996), and such phosphorylation requires myosin II activity (Kuo et al. 2011; Lehman and Morgan 2012). Another study on mouse uterus smooth muscle cells, under chronic stretch during pregnancy demonstrated their ability to sense and mechanically adapt to chronic changes in exogenous forces (Wu et al. 2008). This study further found a positive causality between the pregnancy related chronic stretch and the expression level of dense plaque proteins, FAK and paxillin, as well as increases in the phosphorylation of FAK, paxillin, c-Src and ERK1/2. This demonstrates the ability of smooth muscle cells to sense and adapt to mechanical stress by changing the phosphorylation state of adhesion proteins accordingly, leading to enhanced resilience along the smooth muscle cells (Wu et al. 2008). Such findings could further imply a similar regulatory mechanism in vascular smooth muscle cells coping to chronic mechanical stress due to disease conditions such as hypertension and atherosclerosis.
Podosomes
In addition to dense plaques, vascular smooth muscle cells can form another type of cell–matrix adhesions: podosomes (Fig. 2a, c). Podosomes share a subset of adhesion proteins, scaffolding proteins, signalling proteins, cytoskeleton and the associated regulatory proteins with focal adhesions (Block et al. 2008). Podosomes cons of a dense F-actin core, surrounded by a looser actin meshwork and key actin regulators (Gimona 2008; Joosten et al. 2018; Luxenburg et al. 2007; Tanaka et al. 2015), as well as a surrounding ring of adhesion and scaffolding proteins that connect to a cap structure above the actin core through contractile actomyosin filaments (Burgstaller and Gimona 2005; Cox and Jones 2013; Monypenny et al. 2011; Murphy and Courtneidge 2011). The dense actin core is assembled through ARP2/3, which in turn is activated through Cortactin and WASP in the podosome capping structure (Albiges-Rizo et al. 2009; Destaing et al. 2011; Webb et al. 2006). While Arp2/3 is also the main nucleator for the 2D actin gel of the lamellipodium it indeed has a tendency to form three-dimensional structures when activators are present in certain geometries, as has been demonstrated in vitro by Thery et al. on micropatterns or also found in cyto in certain cell types such as T-cells (Galland et al. 2013; Tabdanov et al. 2015). The adhesion ring again includes a set of key molecules, including integrins, talin, vinculin or filamin (Guiet et al. 2012; Linder et al. 2011; van den Dries et al. 2013). It was suggested that the actomyosin tension in the periphery is allowing the central core to provide the protrusive force that is applied to the surface in a pulsatile manner (Labernadie et al. 2014). Despite this requirement for peripheral actomyosin tension, podosome formation is however promoted by low actomyosin contractility, whereas focal adhesion growth is generally promoted by actomyosin tension (Burridge and Guilluy 2016; Yu et al. 2013), as discussed above. Recent work suggested that this inverse relationship is regulated through microtubules which deliver the RhoGEF, GEF-H1 to the different adhesion sites. Microtubules are connected to talin at podosomes and focal adhesions through a complex of KANK liprins, ELKS and LL5β (Bouchet et al. 2016; Rafiq et al. 2019; Sun et al. 2016). The connection through this complex enables sequestering the RhoGEF, GEF-H1, and hence suppressing actomyosin contractility and promoting podosome formation and/or focal adhesion disassembly. In addition to contractility, also the effect of filamin appears to be inverse for focal adhesions and podosomes. Through inhibition of integrin activity, filamin regulates focal adhesion disassembly (Lynch et al. 2011, 2013; Xu et al. 2010). On the other hand the presence of filamin was reported to be necessary for the formation of podosomes and especially podosome rosettes, although it is not entirely clear how this is reconcilable with its function as integrin inhibitor (Guiet et al. 2012).
Whilst podosomes have the ability to adhere to and degrade extracellular matrix, recent data suggest that they also participate in cellular mechanotransduction (Linder and Wiesner 2016; Parekh and Weaver 2016). Similar to focal adhesions, mechanotransduction by podosomes appears to be bi-directional and the ability to sense the passive forces from a rigid substratum requires podosomes to exert forces onto the substratum. Podosome appearance (Collin et al. 2006; Juin et al. 2013), abundance (Juin et al. 2013; Parekh et al. 2011), and functions in terms of proteolytic ability (Alexander et al. 2008; Parekh et al. 2011) are determined by extracellular stiffnesses. However, whether soft or hard surface is more preferable to podosome formation is debatable and cell type dependent. Vascular smooth muscle cells could form podosome-like protrusions without chemical stimuli on soft 3D matrices (Furmaniak-Kazmierczak et al. 2007) and when placed in a pressure chamber, mimicking stage II hypertension (Kim et al. 2015). In dendritic cells, podosome are preferably formed at pores with lower physical constraint on hard substrate (Baranov et al. 2014). In contrast, endothelial cells form more and larger podosomes on harder stiffnesses (20 kPa) compared to soft stiffness (2 kPa) (Juin et al. 2013). Similarly, in kidney cells, hard surfaces give larger, more persistent, and less dispersed podosomes (Collin et al. 2006). This discrepancy could be due to the differences in the intensities of multiple intracellular signalling pathways, both biochemical and mechanical, which remain elusive and requires further investigation.
Besides extracellular stiffness, extracellular topography is also a determining factor to podosome formation. Apart from stiffness, geometry and topography also matters to the size and duration of podosomes. This appears to be true for smooth muscle as well as non-muscle cells. Vascular smooth muscle cell podosome formation is induced when the cells face microfabricated barriers that mimic the situation of VSMC contacting a stent in vivo (Kim et al. 2015). Dendritic cells reinforce podosome stability on 3D matrices by suppressing Prostaglandin E2 and downstream Rho pathway activity (van den Dries et al. 2012). Miniature rough surface favours large podosome rings in dendritic cells and can extend the podosome lifetime from minutes to hours (Geblinger et al. 2010). As mentioned previously, podosomes also preferably form on soft pores with low physical constraint instead of the surrounding hard substrates (Gawden-Bone et al. 2010). Apart from their mechanosensory properties, podosomes are also mechanical devices exerting forces on the extracellular environment. Importantly, this behaviour is responsive to extracellular stiffnesses as well, since actin polymerization dependent protrusive force generation changes with extracellular stiffness (Collin et al. 2008; Labernadie et al. 2014).
Conclusions
There is strong evidence for a role of extracellular matrix stiffness in cardiovascular disease and the literature regarding the effects of the mechanical stimuli on cardiomyocytes and vascular smooth muscle cells is expanding rapidly. However, there are still many open questions towards the molecular mechanisms behind rigidity sensing and especially the differences between the various cell and adhesion types. Proteomic analysis of muscle cell adhesions similar to the work from non-muscle cells could help shed light on these differences. Moreover, tools and approaches to analyse the behaviour of different integrins [e.g. DNA origami nanoarrays (Hawkes et al. 2019)] or to analyse cellular or molecular forces with high temporal and spatial resolution (such as nanopillars or tension sensors) will help to better understand the specifics of cardiac or smooth muscle cell mechanosensing.
Abbreviations
- CM:
-
Cardiomyocytes
- CF:
-
Cardiac fibroblasts
- VSMC:
-
Vascular smooth muscle cells
- ECM:
-
Extracellular matrix
- CVD:
-
Cardiovascular diseases
- MI:
-
Myocardial infarction
- HCM:
-
Hypertrophic cardiomyopathy
- DCM:
-
Dilated cardiomyopathy
- MMP:
-
Matrix metalloproteinases
- ROS:
-
Reactive oxygen species
- FAK:
-
Focal adhesion kinase
- DGC:
-
Dystrophin-glycoprotein complex
- nmM:
-
Non-muscle myosin
References
Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR (2009) Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci 122:3037–3049. https://doi.org/10.1242/jcs.052704
Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM (2008) Extracellular matrix rigidity promotes invadopodia activity. Curr Biol 18:1295–1299. https://doi.org/10.1016/j.cub.2008.07.090
Awada HK, Hwang MP, Wang Y (2016) Towards comprehensive cardiac repair and regeneration after myocardial infarction: aspects to consider and proteins to deliver. Biomaterials 82:94–112. https://doi.org/10.1016/j.biomaterials.2015.12.025
Bae YH et al (2014) A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal 7:ra57. https://doi.org/10.1126/scisignal.2004838
Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH (1996) Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation 94:3263–3270
Baranov M, Ter Beest M, Reinieren-Beeren I, Cambi A, Figdor CG, van den Bogaart G (2014) Podosomes of dendritic cells facilitate antigen sampling. J Cell Sci 127:1052–1064. https://doi.org/10.1242/jcs.141226
Baudino TA, Carver W, Giles W, Borg TK (2006) Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol 291:H1015–H1026. https://doi.org/10.1152/ajpheart.00023.2006
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA (2017) Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol 19:724–731. https://doi.org/10.1038/ncb3537
Block MR et al (2008) Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol 87:491–506. https://doi.org/10.1016/j.ejcb.2008.02.012
Bouchet BP et al (2016) Talin-KANK1 interaction controls the recruitment of cortical microtubule stabilizing complexes to focal adhesions. Elife 5:e18124. https://doi.org/10.7554/elife.18124
Bouvet M et al (2016) Increased level of phosphorylated desmin and its degradation products in heart failure. Biochem Biophys Rep 6:54–62. https://doi.org/10.1016/j.bbrep.2016.02.014
Brown RD, Ambler SK, Mitchell MD, Long CS (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45:657–687. https://doi.org/10.1146/annurev.pharmtox.45.120403.095802
Bujak M et al (2009) Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 105:973–983. https://doi.org/10.1161/CIRCRESAHA.109.199471
Burgstaller G, Gimona M (2005) Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 288:H3001–H3005. https://doi.org/10.1152/ajpheart.01002.2004
Burridge K, Connell L (1983) A new protein of adhesion plaques and ruffling membranes. J Cell Biol 97:359–367. https://doi.org/10.1083/jcb.97.2.359
Burridge K, Guilluy C (2016) Focal adhesions, stress fibers and mechanical tension. Exp Cell Res 343:14–20. https://doi.org/10.1016/j.yexcr.2015.10.029
Chang CW, Dalgliesh AJ, Lopez JE, Griffiths LG (2016) Cardiac extracellular matrix proteomics: challenges, techniques, and clinical implications. Proteom Clin Appl 10:39–50. https://doi.org/10.1002/prca.201500030
Chaterji S et al (2014) Synergistic effects of matrix nanotopography and stiffness on vascular smooth muscle cell function. Tissue Eng Part A 20:2115–2126. https://doi.org/10.1089/ten.tea.2013.0455
Clemen CS et al (2015) The toxic effect of R350P mutant desmin in striated muscle of man and mouse. Acta Neuropathol 129:297–315. https://doi.org/10.1007/s00401-014-1363-2
Collin O, Tracqui P, Stephanou A, Usson Y, Clement-Lacroix J, Planus E (2006) Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J Cell Sci 119:1914–1925. https://doi.org/10.1242/jcs.02838
Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N (2008) Self-organized podosomes are dynamic mechanosensors. Curr Biol 18:1288–1294. https://doi.org/10.1016/j.cub.2008.07.046
Constantin B (2014) Dystrophin complex functions as a scaffold for signalling proteins. Biochem Biophys Acta 1838:635–642. https://doi.org/10.1016/j.bbamem.2013.08.023
Cox S, Jones GE (2013) Imaging cells at the nanoscale. Int J Biochem Cell Biol 45:1669–1678. https://doi.org/10.1016/j.biocel.2013.05.010
de Beaufort HWL et al (2018) Comparative analysis of porcine and human thoracic aortic stiffness. Eur J Vasc Endovasc Surg 55:560–566. https://doi.org/10.1016/j.ejvs.2017.12.014
Destaing O, Block MR, Planus E, Albiges-Rizo C (2011) Invadosome regulation by adhesion signaling. Curr Opin Cell Biol 23:597–606. https://doi.org/10.1016/j.ceb.2011.04.002
Dorland YL, Huveneers S (2017) Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci 74:279–292. https://doi.org/10.1007/s00018-016-2325-8
Draeger A, Amos WB, Ikebe M, Small JV (1990) The cytoskeletal and contractile apparatus of smooth muscle: contraction bands and segmentation of the contractile elements. J Cell Biol 111:2463–2473. https://doi.org/10.1083/jcb.111.6.2463
Elosegui-Artola A et al (2016) Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol 18:540–548. https://doi.org/10.1038/ncb3336
Elosegui-Artola A, Trepat X, Roca-Cusachs P (2018) Control of mechanotransduction by molecular clutch dynamics. Trends Cell Biol 28:356–367. https://doi.org/10.1016/j.tcb.2018.01.008
Engler AJ et al (2008) Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121:3794–3802. https://doi.org/10.1242/jcs.029678
Ervasti JM (2003) Costameres: the Achilles’ heel of Herculean muscle. J Biol Chem 278:13591–13594. https://doi.org/10.1074/jbc.R200021200
Etoh T et al (2001) Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol 281:H987–H994. https://doi.org/10.1152/ajpheart.2001.281.3.H987
Feramisco JR, Burridge K (1980) A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem 255:1194–1199
Flick MJ, Konieczny SF (2000) The muscle regulatory and structural protein MLP is a cytoskeletal binding partner of betaI-spectrin. J Cell Sci 113(Pt 9):1553–1564
Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Cote GP (2007) Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res 100:1328–1336. https://doi.org/10.1161/CIRCRESAHA.106.147744
Galland R, Leduc P, Guerin C, Peyrade D, Blanchoin L, Thery M (2013) Fabrication of three-dimensional electrical connections by means of directed actin self-organization. Nat Mater 12:416–421. https://doi.org/10.1038/nmat3569
Gawden-Bone C, Zhou Z, King E, Prescott A, Watts C, Lucocq J (2010) Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J Cell Sci 123:1427–1437. https://doi.org/10.1242/jcs.056515
Geblinger D, Addadi L, Geiger B (2010) Nano-topography sensing by osteoclasts. J Cell Sci 123:1503–1510. https://doi.org/10.1242/jcs.060954
Geiger B (1979) A 130 K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell 18:193–205
Geisler N, Weber K (1988) Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J 7:15–20
Gimona M (2008) The microfilament system in the formation of invasive adhesions. Semin Cancer Biol 18:23–34. https://doi.org/10.1016/j.semcancer.2007.08.005
Gozna ER, Marble AE, Shaw AJ, Winter DA (1973) Mechanical properties of the ascending thoracic aorta of man. Cardiovasc Res 7:261–265. https://doi.org/10.1093/cvr/7.2.261
Grant CA, Twigg PC (2013) Pseudostatic and dynamic nanomechanics of the tunica adventitia in elastic arteries using atomic force microscopy. ACS Nano 7:456–464. https://doi.org/10.1021/nn304508x
Guiet R et al (2012) Macrophage mesenchymal migration requires podosome stabilization by filamin A. J Biol Chem 287:13051–13062. https://doi.org/10.1074/jbc.M111.307124
Gunst SJ, Tang DD (2000) The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 15:600–616
Haining AWM et al (2018) Mechanotransduction in talin through the interaction of the R8 domain with DLC1. PLoS Biol 16:e2005599. https://doi.org/10.1371/journal.pbio.2005599
Hanna MA et al (2014) Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol 117:616–623. https://doi.org/10.1152/japplphysiol.01296.2013
Hartman CD, Isenberg BC, Chua SG, Wong JY (2016) Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci USA 113:11190–11195. https://doi.org/10.1073/pnas.1611324113
Hawkes W et al (2019) Probing the nanoscale organisation and multivalency of cell surface receptors: DNA origami nanoarrays for cellular studies with single-molecule control. Faraday Discuss. https://doi.org/10.1039/c9fd00023b
Hazeltine LB et al (2012) Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol 2012:508294. https://doi.org/10.1155/2012/508294
Hein S, Kostin S, Heling A, Maeno Y, Schaper J (2000) The role of the cytoskeleton in heart failure. Cardiovasc Res 45:273–278. https://doi.org/10.1016/s0008-6363(99)00268-0
Hemmasizadeh A, Autieri M, Darvish K (2012) Multilayer material properties of aorta determined from nanoindentation tests. J Mech Behav Biomed Mater 15:199–207. https://doi.org/10.1016/j.jmbbm.2012.06.008
Hermida N et al (2009) A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res 81:601–609. https://doi.org/10.1093/cvr/cvn315
Hersch N, Wolters B, Dreissen G, Springer R, Kirchgessner N, Merkel R, Hoffmann B (2013) The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening. Biol Open 2:351–361. https://doi.org/10.1242/bio.20133830
Horton ER et al (2015) Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 17:1577–1587. https://doi.org/10.1038/ncb3257
Hu S, Tee YH, Kabla A, Zaidel-Bar R, Bershadsky A, Hersen P (2015) Structured illumination microscopy reveals focal adhesions are composed of linear subunits. Cytoskeleton (Hoboken) 72:235–245. https://doi.org/10.1002/cm.21223
Hughes WM Jr et al (2008) Role of copper and homocysteine in pressure overload heart failure. Cardiovasc Toxicol 8:137–144. https://doi.org/10.1007/s12012-008-9021-3
Isenberg BC, Dimilla PA, Walker M, Kim S, Wong JY (2009) Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J 97:1313–1322. https://doi.org/10.1016/j.bpj.2009.06.021
Iskratsch T et al (2013) FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev Cell 27:545–559. https://doi.org/10.1016/j.devcel.2013.11.003
Iskratsch T, Wolfenson H, Sheetz MP (2014) Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 15:825–833. https://doi.org/10.1038/nrm3903
Jani K, Schock F (2009) Molecular mechanisms of mechanosensing in muscle development. Dev Dyn 238:1526–1534. https://doi.org/10.1002/dvdy.21972
Jian Z et al (2014) Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal 7:ra27. https://doi.org/10.1126/scisignal.2005046
Joosten B, Willemse M, Fransen J, Cambi A, van den Dries K (2018) Super-resolution correlative light and electron microscopy (SR-CLEM) reveals novel ultrastructural insights into dendritic cell podosomes. Front Immunol 9:1908. https://doi.org/10.3389/fimmu.2018.01908
Juin A et al (2013) Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biol Cell 105:46–57. https://doi.org/10.1111/boc.201200037
Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468:580–584. https://doi.org/10.1038/nature09621
Kawada T et al (2003) Sarcolemmal fragility secondary to the degradation of dystrophin in dilated cardiomyopathy, as estimated by electron microscopy. Exp Clin Cardiol 8:67–70
Kelly T, Molony L, Burridge K (1987) Purification of two smooth muscle glycoproteins related to integrin. Distribution in cultured chicken embryo fibroblasts. J Biol Chem 262:17189–17199
Khamdaeng T, Luo J, Vappou J, Terdtoon P, Konofagou EE (2012) Arterial stiffness identification of the human carotid artery using the stress-strain relationship in vivo. Ultrasonics 52:402–411. https://doi.org/10.1016/j.ultras.2011.09.006
Khanafer K, Duprey A, Zainal M, Schlicht M, Williams D, Berguer R (2011) Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J Thorac Cardiovasc Surg 142:682–686. https://doi.org/10.1016/j.jtcvs.2010.09.068
Kim NY, Kohn JC, Huynh J, Carey SP, Mason BN, Vouyouka AG, Reinhart-King CA (2015) Biophysical induction of vascular smooth muscle cell podosomes. PLoS ONE 10:e0119008. https://doi.org/10.1371/journal.pone.0119008
Klapholz B, Brown NH (2017) Talin—the master of integrin adhesions. J Cell Sci 130:2435–2446. https://doi.org/10.1242/jcs.190991
Klein EA et al (2009) Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19:1511–1518. https://doi.org/10.1016/j.cub.2009.07.069
Knoll R et al (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111:943–955
Kohn JC, Lampi MC, Reinhart-King CA (2015) Age-related vascular stiffening: causes and consequences. Front Genet 6:112. https://doi.org/10.3389/fgene.2015.00112
Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C (2009) Demonstration of catch bonds between an integrin and its ligand. J Cell Biol 185:1275–1284. https://doi.org/10.1083/jcb.200810002
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71:549–574. https://doi.org/10.1007/s00018-013-1349-6
Kumar A et al (2016) Correction: talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J Cell Biol 214:231. https://doi.org/10.1083/jcb.20151001207062016c
Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM (2011) Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13:383–393. https://doi.org/10.1038/ncb2216
Labernadie A et al (2014) Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nat Commun 5:5343. https://doi.org/10.1038/ncomms6343
Lakatta EG, Mitchell JH, Pomerance A, Rowe GG (1987) Human aging: changes in structure and function. J Am Coll Cardiol 10:42A–47A
Lapidos KA, Kakkar R, McNally EM (2004) The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res 94:1023–1031. https://doi.org/10.1161/01.RES.0000126574.61061.25
Laurent S et al (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37:1236–1241
Le S, Yu M, Hovan L, Zhao Z, Ervasti J, Yan J (2018a) Dystrophin as a molecular shock absorber. ACS Nano 12:12140–12148. https://doi.org/10.1021/acsnano.8b05721
Le V et al (2018b) Syndecan-1 in mechanosensing of nanotopological cues in engineered materials. Biomaterials 155:13–24. https://doi.org/10.1016/j.biomaterials.2017.11.007
Leal J, Luengo-Fernandez R, Gray A, Petersen S, Rayner M (2006) Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J 27:1610–1619. https://doi.org/10.1093/eurheartj/ehi733
Legate KR, Fassler R (2009) Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 122:187–198. https://doi.org/10.1242/jcs.041624
Lehman W, Morgan KG (2012) Structure and dynamics of the actin-based smooth muscle contractile and cytoskeletal apparatus. J Muscle Res Cell Motil 33:461–469. https://doi.org/10.1007/s10974-012-9283-z
Li A, Ponten F, dos Remedios CG (2012) The interactome of LIM domain proteins: the contributions of LIM domain proteins to heart failure and heart development. Proteomics 12:203–225. https://doi.org/10.1002/pmic.201100492
Li J, Mkrtschjan MA, Lin YH, Russell B (2016) Variation in stiffness regulates cardiac myocyte hypertrophy via signaling pathways. Can J Physiol Pharmacol 94:1178–1186. https://doi.org/10.1139/cjpp-2015-0578
Linder S, Wiesner C (2016) Feel the force: podosomes in mechanosensing. Exp Cell Res 343:67–72. https://doi.org/10.1016/j.yexcr.2015.11.026
Linder S, Wiesner C, Himmel M (2011) Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 27:185–211. https://doi.org/10.1146/annurev-cellbio-092910-154216
Liu J, Das M, Yang J, Ithychanda SS, Yakubenko VP, Plow EF, Qin J (2015) Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol 22:383–389. https://doi.org/10.1038/nsmb.2999
Lockhart M, Wirrig E, Phelps A, Wessels A (2011) Extracellular matrix and heart development. Birth Defects Res A 91:535–550. https://doi.org/10.1002/bdra.20810
Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L (2007) The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS ONE 2:e179. https://doi.org/10.1371/journal.pone.0000179
Lynch CD, Gauthier NC, Biais N, Lazar AM, Roca-Cusachs P, Yu CH, Sheetz MP (2011) Filamin depletion blocks endoplasmic spreading and destabilizes force-bearing adhesions. Mol Biol Cell 22:1263–1273. https://doi.org/10.1091/mbc.E10-08-0661
Lynch CD, Lazar AM, Iskratsch T, Zhang X, Sheetz MP (2013) Endoplasmic spreading requires coalescence of vimentin intermediate filaments at force-bearing adhesions. Mol Biol Cell 24:21–30. https://doi.org/10.1091/mbc.E12-05-0377
Maki JM (2009) Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol 24:651–660. https://doi.org/10.14670/HH-24.651
Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, Sheetz M (2011) Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol 9:e1001223. https://doi.org/10.1371/journal.pbio.1001223
Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE (1994) The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol 23:1204–1208
Matsumoto T, Hayashi K (1996) Stress and strain distribution in hypertensive and normotensive rat aorta considering residual strain. J Biomech Eng 118:62–73. https://doi.org/10.1115/1.2795947
McCormick RJ, Thomas DP (1998) Collagen crosslinking in the heart: relationship to development and function. Basic Appl Myol 8:143–150
McCormick RJ, Musch TI, Bergman BC, Thomas DP (1994) Regional differences in LV collagen accumulation and mature cross-linking after myocardial infarction in rats. Am J Physiol 266:H354–H359. https://doi.org/10.1152/ajpheart.1994.266.1.H354
McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung KH, Plant AL (2007) The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys J 92:1759–1769. https://doi.org/10.1529/biophysj.106.089003
Meacci G et al (2016) Alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell 27:3471–3479. https://doi.org/10.1091/mbc.E16-02-0107
Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1:761–772
Monypenny J, Chou HC, Banon-Rodriguez I, Thrasher AJ, Anton IM, Jones GE, Calle Y (2011) Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur J Cell Biol 90:198–204. https://doi.org/10.1016/j.ejcb.2010.05.009
Morgan MR, Humphries MJ, Bass MD (2007) Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8:957–969. https://doi.org/10.1038/nrm2289
Murphy DA, Courtneidge SA (2011) The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 12:413–426. https://doi.org/10.1038/nrm3141
Nakamura A, Yoshida K, Takeda S, Dohi N, Ikeda S (2002) Progression of dystrophic features and activation of mitogen-activated protein kinases and calcineurin by physical exercise, in hearts of mdx mice. FEBS Lett 520:18–24
North AJ, Galazkiewicz B, Byers TJ, Glenney JR Jr, Small JV (1993) Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle. J Cell Biol 120:1159–1167. https://doi.org/10.1083/jcb.120.5.1159
Oakes PW, Beckham Y, Stricker J, Gardel ML (2012) Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol 196:363–374. https://doi.org/10.1083/jcb.201107042
Oakes PW et al (2018) Lamellipodium is a myosin-independent mechanosensor. Proc Natl Acad Sci U S A 115:2646–2651. https://doi.org/10.1073/pnas.1715869115
Oliviero P et al (2000) Expression of laminin alpha2 chain during normal and pathological growth of myocardium in rat and human. Cardiovasc Res 46:346–355. https://doi.org/10.1016/s0008-6363(00)00034-1
Pandey P et al (2018) Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev Cell 44(326–336):e323. https://doi.org/10.1016/j.devcel.2017.12.024
Parekh A, Weaver AM (2016) Regulation of invadopodia by mechanical signaling. Exp Cell Res 343:89–95. https://doi.org/10.1016/j.yexcr.2015.10.038
Parekh A et al (2011) Sensing and modulation of invadopodia across a wide range of rigidities. Biophys J 100:573–582. https://doi.org/10.1016/j.bpj.2010.12.3733
Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM (2010) Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188:877–890. https://doi.org/10.1083/jcb.200906012
Pavalko FM, Adam LP, Wu MF, Walker TL, Gunst SJ (1995) Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction. Am J Physiol 268:C563–C571. https://doi.org/10.1152/ajpcell.1995.268.3.C563
Peyton SR, Putnam AJ (2005) Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 204:198–209. https://doi.org/10.1002/jcp.20274
Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ (2008) The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials 29:2597–2607. https://doi.org/10.1016/j.biomaterials.2008.02.005
Plotnikov SV, Pasapera AM, Sabass B, Waterman CM (2012) Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151:1513–1527. https://doi.org/10.1016/j.cell.2012.11.034
Rafiq NBM et al (2019) A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater 18:638–649. https://doi.org/10.1038/s41563-019-0371-y
Renley BA, Rybakova IN, Amann KJ, Ervasti JM (1998) Dystrophin binding to nonmuscle actin. Cell Motil Cytoskeleton 41:264–270
Ribeiro AJ et al (2015) Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A 112:12705–12710. https://doi.org/10.1073/pnas.1508073112
Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S (2014) Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res 114:872–888. https://doi.org/10.1161/CIRCRESAHA.114.302533
Robison P et al (2016) Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352:aaf0659. https://doi.org/10.1126/science.aaf0659
Roca-Cusachs P, Iskratsch T, Sheetz MP (2012) Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 125:3025–3038. https://doi.org/10.1242/jcs.095794
Rodriguez AG, Han SJ, Regnier M, Sniadecki NJ (2011) Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J 101:2455–2464. https://doi.org/10.1016/j.bpj.2011.09.057
Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ (2014) Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J Biomech Eng 136:051005. https://doi.org/10.1115/1.4027145
Schlatmann TJ, Becker AE (1977) Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39:13–20
Schleicher ED, Wagner E, Nerlich AG (1997) Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest 99:457–468. https://doi.org/10.1172/JCI119180
Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ (1996) The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39:946–951
Small JV (1985) Geometry of actin-membrane attachments in the smooth muscle cell: the localisations of vinculin and alpha-actinin. EMBO J 4:45–49
Soto-Ribeiro M et al (2019) beta1D integrin splice variant stabilizes integrin dynamics and reduces integrin signaling by limiting paxillin recruitment. J Cell Sci. https://doi.org/10.1242/jcs.224493
Stevenson S, Rothery S, Cullen MJ, Severs NJ (1997) Dystrophin is not a specific component of the cardiac costamere. Circ Res 80:269–280
Sugita S, Mizutani E, Hozaki M, Nakamura M, Matsumoto T (2019) Photoelasticity-based evaluation of cellular contractile force for phenotypic discrimination of vascular smooth muscle cells. Sci Rep 9:3960. https://doi.org/10.1038/s41598-019-40578-7
Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD 3rd (2014) Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther 5:14. https://doi.org/10.1186/scrt403
Sun Z et al (2016) Kank2 activates talin, reduces force transduction across integrins and induces central adhesion formation. Nat Cell Biol 18:941–953. https://doi.org/10.1038/ncb3402
Swaminathan V, Waterman CM (2016) The molecular clutch model for mechanotransduction evolves. Nat Cell Biol 18:459–461. https://doi.org/10.1038/ncb3350
Tabdanov E et al (2015) Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells. Integr Biol (Camb) 7:1272–1284. https://doi.org/10.1039/c5ib00032g
Tanaka H, Wang HH, Thatcher SE, Hagiwara H, Takano-Ohmuro H, Kohama K (2015) Electron microscopic examination of podosomes induced by phorbol 12, 13 dibutyrate on the surface of A7r5 cells. J Pharmacol Sci 128:78–82. https://doi.org/10.1016/j.jphs.2015.03.002
Tang DD (2008) Intermediate filaments in smooth muscle. Am J Physiol Cell Physiol 294:C869–C878. https://doi.org/10.1152/ajpcell.00154.2007
Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK (1991) Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res 68:734–744
Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D (1997) Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol 29:2107–2124
Tojkander S, Gateva G, Lappalainen P (2012) Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci 125:1855–1864. https://doi.org/10.1242/jcs.098087
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118:1021–1040. https://doi.org/10.1161/CIRCRESAHA.115.306565
Turner CE, Kramarcy N, Sealock R, Burridge K (1991) Localization of paxillin, a focal adhesion protein, to smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. Exp Cell Res 192:651–655
van Deel ED et al (2017) In vitro model to study the effects of matrix stiffening on Ca(2+) handling and myofilament function in isolated adult rat cardiomyocytes. J Physiol 595:4597–4610. https://doi.org/10.1113/JP274460
van den Dries K et al (2012) Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol Life Sci 69:1889–1901. https://doi.org/10.1007/s00018-011-0908-y
van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A (2013) Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun 4:1412. https://doi.org/10.1038/ncomms2402
Van Itallie CM, Tietgens AJ, Aponte A, Fredriksson K, Fanning AS, Gucek M, Anderson JM (2014) Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. J Cell Sci 127:885–895. https://doi.org/10.1242/jcs.140475
Vijayakumar V et al (2015) Tyrosine phosphorylation of WIP releases bound WASP and impairs podosome assembly in macrophages. J Cell Sci 128:251–265. https://doi.org/10.1242/jcs.154880
Wagenseil JE, Mecham RP (2009) Vascular extracellular matrix and arterial mechanics. Physiol Rev 89:957–989. https://doi.org/10.1152/physrev.00041.2008
Wakayama Y, Shibuya S, Jimi T, Takeda A, Oniki H (1993) Size and localization of dystrophin molecule: immunoelectron microscopic and freeze etching studies of muscle plasma membranes of murine skeletal myofibers. Acta Neuropathol 86:567–577
Wang Z, Pavalko FM, Gunst SJ (1996) Tyrosine phosphorylation of the dense plaque protein paxillin is regulated during smooth muscle contraction. Am J Physiol 271:C1594–C1602. https://doi.org/10.1152/ajpcell.1996.271.5.C1594
Ward M, Iskratsch T (2019) Mix and (mis-)match—the mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart. Biochim Biophys Acta Mol Cell Res. https://doi.org/10.1016/j.bbamcr.2019.01.017
Webb BA, Eves R, Mak AS (2006) Cortactin regulates podosome formation: roles of the protein interaction domains. Exp Cell Res 312:760–769. https://doi.org/10.1016/j.yexcr.2005.11.032
Weber KT, Sun Y, Tyagi SC, Cleutjens JP (1994) Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol 26:279–292. https://doi.org/10.1006/jmcc.1994.1036
Winograd-Katz SE, Fassler R, Geiger B, Legate KR (2014) The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol 15:273–288. https://doi.org/10.1038/nrm3769
Wolfenson H et al (2016) Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat Cell Biol 18:33–42. https://doi.org/10.1038/ncb3277
Wu X, Morgan KG, Jones CJ, Tribe RM, Taggart MJ (2008) Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodelling. J Cell Mol Med 12:1360–1373. https://doi.org/10.1111/j.1582-4934.2008.00306.x
Xu Y et al (2010) Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med 207:2421–2437. https://doi.org/10.1084/jem.20100433
Yao M et al (2016) The mechanical response of talin. Nat Commun 7:11966. https://doi.org/10.1038/ncomms11966
Yates LA, Fuzery AK, Bonet R, Campbell ID, Gilbert RJ (2012) Biophysical analysis of Kindlin-3 reveals an elongated conformation and maps integrin binding to the membrane-distal beta-subunit NPXY motif. J Biol Chem 287:37715–37731. https://doi.org/10.1074/jbc.M112.415208
Young JL, Kretchmer K, Ondeck MG, Zambon AC, Engler AJ (2014) Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci Rep 4:6425. https://doi.org/10.1038/srep06425
Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, Sheetz MP (2013) Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep 5:1456–1468. https://doi.org/10.1016/j.celrep.2013.10.040
Zhang W, Gunst SJ (2008) Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc 5:32–39. https://doi.org/10.1513/pats.200704-048VS
Zhou J, Fung YC (1997) The degree of nonlinearity and anisotropy of blood vessel elasticity. Proc Natl Acad Sci U S A 94:14255–14260. https://doi.org/10.1073/pnas.94.26.14255
Acknowledgements
T.I. was supported by a British Heart Foundation Intermediate Basic Science Research Fellowship (FS/14/30/30917) and a BBSRC New Investigator Award (BB/S001123/1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sit, B., Gutmann, D. & Iskratsch, T. Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing. J Muscle Res Cell Motil 40, 197–209 (2019). https://doi.org/10.1007/s10974-019-09529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-019-09529-7