Abstract
Purpose
To assess whether preimplantation genetic testing for aneuploidies (PGT-A) at the blastocyst stage improves clinical outcomes compared with transfer of embryos without PGT-A in poor ovarian response (POR) patients.
Methods
Retrospective cohort study of IVF cycles from 2016 to 2019 at a single academic fertility center. IVF cycles with POR and four or fewer oocytes retrieved were stratified into PGT-A (n = 241) and non-PGT (n = 112) groups. In PGT-A cycles, trophectoderm biopsy, next-generation sequencing with 24-chromosome screening, and single euploid frozen embryo transfer were performed. In non-PGT cycles, fresh or frozen transfer of untested embryos on day 3 or 5 was performed. Main outcomes included live birth rate and miscarriage rate per retrieval.
Result(s)
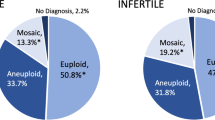
Patients who underwent PGT-A cycles were significantly less likely to reach embryo transfer compared with those who underwent non-PGT cycles (13.7% vs 70.6%). The live birth rate per retrieval did not differ between the PGT-A and non-PGT groups (6.6% vs 5.4%). Overall, the miscarriage rate was low. The PGT-A group demonstrated a significantly lower miscarriage rate per retrieval (0.4% vs 3.6%) as well as per pregnancy (5.9% vs 40.0%) compared with the non-PGT group. The number needed to treat to avoid one clinical miscarriage was 31 PGT-A cycles.
Conclusion(s)
PGT-A did not improve live birth rate per retrieval in POR patients with four or fewer oocytes retrieved. PGT-A was associated with a lower miscarriage rate; however, a fairly large number of PGT-A cycles were needed to prevent one miscarriage.

Similar content being viewed by others

Data availability
The material contained in the manuscript has not been published, has not been submitted, or is not being submitted elsewhere for publication.
Code availability
N/A.
References
Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril. 2015;104:612–19.e3.
Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. 2015;30:315–22.
Oudendijk JF, Yarde F, Eijkemans MJ, Broekmans FJ, Broer SL. The poor responder in IVF: is the prognosis always poor? A systematic review. Hum Reprod Update. 2012;18:1–11.
Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–703.
Munné S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod BioMed Online. 2007;14:628–34.
Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23:2806–12.
Sermon K, Capalbo A, Cohen J, Coonen E, De Rycke M, De Vos A, et al. The why, the how and the when of PGS 2.0: current practices and expert opinions of fertility specialists, molecular biologists, and embryologists. Mol Hum Reprod. 2016;22:845–57.
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–9.e7.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24.
Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100–7.e1.
Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703.
Glujovsky DFC, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. In Cochrane Database Syst Rev. 2016;6:CD002118.
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–24.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–8.
Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107:1122–9.
Munné S, Alikani M, Ribustello L, Colls P, Martínez-Ortiz PA, McCulloh DH, et al. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32:743–9.
Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36:629–36.
Papathanasiou A. Implementing the ESHRE 'poor responder’ criteria in research studies: methodological implications. Hum Reprod. 2014;29:1835–8.
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16:113–30.
Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30:473–83.
Morin SJ, Patounakis G, Juneau CR, Neal SA, Scott RT, Seli E. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: a quantitative but not qualitative reduction in performance. Hum Reprod 2018.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8.
Kushnir VA, Vidali A, Barad DH, Gleicher N. The status of public reporting of clinical outcomes in assisted reproductive technology. Fertil Steril. 2013;100:736–41.
Kulak D, Jindal SK, Oh C, Morelli SS, Kratka S, McGovern PG. Reporting in vitro fertilization cycles to the Society for Assisted Reproductive Technology database: where have all the cycles gone? Fertil Steril. 2016;105:927–31.e3.
Author information
Authors and Affiliations
Contributions
J.D. performed study design, execution, statistical analysis, and wrote the manuscript. H.H., Q.Z., A.N., and S.A. coordinated data collection and statistical analysis. B.B. and R.L. contributed interpretation of the data and edit of the manuscript. We thank Stephanie A. Leonard for assistance in statistics. All authors reviewed the manuscript and provided critical feedback and discussion.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have conflicts of interest.
Ethics approval
This research is approved by IRB at Stanford University.
Consent to participate
Waivered.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The work was done in Stanford Medicine Fertility and Reproductive Health Services.
Electronic supplementary material
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Deng, J., Hong, H.Y., Zhao, Q. et al. Preimplantation genetic testing for aneuploidy in poor ovarian responders with four or fewer oocytes retrieved. J Assist Reprod Genet 37, 1147–1154 (2020). https://doi.org/10.1007/s10815-020-01765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01765-y