Abstract
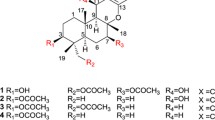
Roridin E is a well-known macrocyclic trichothecene mycotoxin possessing potent antiproliferative activity against cancer cell lines. 12′-Hydroxyroridin E was isolated from a marine-derived fungus, Myrothecium roridum 98F42. The cytotoxicities of these two compounds were tested against human monocytic THP-1, human promyelocytic leukemia HL-60, and Chinese hamster V79 cells, and roridin E exhibited more than 1000-fold stronger cytotoxicity than its 12′-OH derivative; therefore, it was suggested that the 12′-position is closely involved in the cytotoxicity of these compounds.




Similar content being viewed by others
References
Abbas HK, Tak H, Boyette CD, Shier WT, Jarvis BB (2001) Macrocyclic trichothecenes are undetectable in kudzu (Pueraria montana) plants treated with a high-producing isolate of Myrothecium verrucaria. Phytochemistry 58:269–276
Abbas HK, Johnson BB, Shier WT, Tak H, Jarvis BB, Boyette CD (2002) Phytotoxicity and mammalian cytotoxicity of macrocyclic trichothecene mycotoxins from Myrothecium verrucaria. Phytochemistry 59:309–313
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium–based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47:939–942
Garcia CC, Rosso ML, Bertoni MD, Maier MS, Damonte EB (2002) Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia. Planta Med 68:209–212
Hughes BJ, Hsieh GC, Jarvis BB, Sharma RP (1989) Effects of macrocyclic trichothecene mycotoxins on the murine immune system. Arch Environ Contam Toxicol 18:388–395
Isaka M, Punya J, Lertwerawat Y, Tanticharoen M, Thebtaranonth Y (1999) Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria. J Nat Prod 62:329–331
Namikoshi M, Kobayashi H, Yoshimoto T, Meguro S, Akano K (2000) Isolation and characterization of bioactive metabolites from marine-derived filamentous fungi collected from tropical and sub-tropical coral reefs. Chem Pharm Bull 48:1452–1457
Namikoshi M, Akano K, Meguro S, Kasuga I, Mine Y, Takahashi T, Kobayashi H (2001) A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine-derived fungus Myrothecium roridum collected in Palau. J Nat Prod 64:396–398
Sakakibara Y, Saito I, Ichinoseki K, Oda T, Kaneko M, Saito H, Kodama M, Sato Y (1991) Effects of diethylstilbestrol and its methyl ethers on aneuploidy induction and microtubule distribution in Chinese hamster V79 cells. Mutant Res 263:269–276
Sato Y, Sakakibara Y, Oda T, Aizu-Yokota E, Ichinoseki I (1992) Effects of estradiol and ethynylestradiol on microtubule distribution in Chinese hamster V79 cells. Chem Pharm Bull 40:182–184
Smitka TA, Bunge RH, Bloem RJ, French JC (1984) Two new trichothecenes, PD 113, 325 and PD 113, 326. J Antibiot 37:823–828
Traxler P, Zurcher W, Tamm C (1970) Structure of the antibiotic Roridin E. Helv Chim Acta 53:2071–2085
Xu J, Takasaki A, Kobayashi H, Oda T, Yamada J, Mangindaan REP, Ukai K, Nagai H, Namikoshi M (2006) Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J Antibiot 59:451–455
Zhang HJ, Tamez PA, Aydogmus Z, Tan GT, Saikawa Y, Hashimoto K, Nakata M, Hung NV, Xuan LT, Cuong NM, Soejarto DD, Pezzuto JM, Fong HH (2002) Antimalarial agents form plants. III. Trichothecenes form Ficus fistulosa and Rhaphidophora decursiva. Planta Med 68:1088–1091
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas 17035029 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to M. N. We thank Ms. M. Endo and A. Fujita for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oda, T., Xu, J., Ukai, K. et al. 12′-Hydroxyl group remarkably reduces Roridin E cytotoxicity. Mycoscience 51, 317–320 (2010). https://doi.org/10.1007/s10267-010-0035-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10267-010-0035-x