Abstract
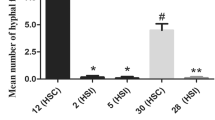
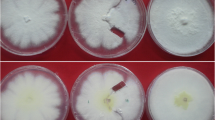
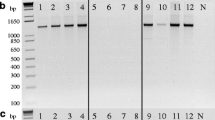
This study evaluates the effectiveness of using single-protoplast isolates (SPIs) to study the mating phenomena of Rhizoctonia solani AG-1 IC and IA. SPIs obtained from three field isolates (F-1, Rh28, and RO2) of AG-1 IC were paired with representative single-basidiospore isolate (SBI)-M1/-M2 testers, each from their own field isolates, or paired in all possible combinations. Tufts were formed between SPIs and SBI-M1/-M2 testers and between SPIs-M1 and -M2. The separation ratios of SPIs-M1 and -M2 were approximately 1:1, which were similar to the results obtained with SBIs. SPIs obtained from three isolates (GNSD, R59, and Tr8) of AG-1 IA, which failed to form basidiospores, were paired in all possible combinations. Although no tufts formed among SPIs from Tr8 and R59, tufts did form between SPIs from GNSD. SPIs from GNSD were separated into homokaryotic (-M1 or -M2) and heterokaryotic isolates, and the separation ratio of -M1 and -M2 was also around 1:1. Amplified fragment length polymorphism (AFLP) phenotypes of the tuft isolates formed between GNSD SPIs-M1 and -M2 suggested that these tuft isolates were all heterokaryotic. These results indicate that all three isolates of AG-1 IC and one isolate GNSD of AG-1 IA are heterokaryotic, and that the other two isolates of Tr8 and R59 of AG-1 IA are homokaryotic. Single-protoplast isolates are effective for studies of the mating phenomena of isolates belonging to different AGs of R. solani that could not form a perfect stage.
Similar content being viewed by others
References
Adams GC (1988) Thanatephorus cucumeris (Rhizoctonia solani), a species complex of wide host range. In: Ingram DS, Williams PH (eds) Advances in plant pathology, vol 6: Genetics of plant pathogenic fungi. Academic Press, London, pp 535–552
Adams GC, Butler EE (1982) A re-interpretation of the sexuality of Thanatephorus cucumeris anastomosis group four. Mycologia 74:793–800
Anderson NA (1982) The genetics and pathology of Rhizoctonia solani. Annu Rev Phytopathol 20:329–347
Anderson NA, Stretton HM, Groth JV, Flentje NT (1972) Genetics of heterokaryosis in Thanatephorus cucumeris. Phytopathology 62:1057–1065
Carling DE (1996) Grouping in Rhizoctonia solani by hyphal anastomosis reaction. In: Sneh B, Jabaji-Hare S, Neate S, Dijst G (eds) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Kluwer, Dordrecht, pp 37–47
Carling DE, Kuninaga S (1990) DNA based-sequence homology in Rhizoctonia solani Kuhn: inter-and intra-group relatedness of anastomosis group-9. Phytopathology 80:1362–1364
Carling DE, Pope EJ, Brainard KA, Carter DA (1999) Characterization of mycorrhizal isolates of Rhizoctonia solani from an orchard, including AG-12, a new anastomosis group. Phytopathology 89:942–946
Carling DE, Baird RE, Gitaitis RD, Brainard KA, Kuninaga S (2002) Characterization of AG-13, a newly reported anastomosis group of Rhizoctonia solani. Phytopathology 92:893–899
Carling DE, Kuninaga S, Brainard KA (2003) Hyphal anastomosis reactions, rDNA-internal transcribed spacers, and virulence levels among subsets of Rhizoctonia solani anastomosis group-2 (AG-2) and AG-BI. Phytopathology 92:43–50
Cubeta MA, Vilgalys RJ (1997) Population biology of the Rhizoctonia solani complex. Phytopathology 87:480–484
Hashiba T, Yamada M (1982) Formation and purification of protoplasts from Rhizoctonia solani. Phytopathology 72:849–853
Julian MC, Debets F, Keijer J (1996) Independence of sexual and vegetative incompatibility mechanisms of Thanatephorus cucumeris (Rhizoctonia solani) anastomosis group 1. Phytopathology 86:566–574
Julian MC, Dullemans AM, Silfhout C, Keijer J (1997) Nuclear behavior in homokaryotic and heterokaryotic fruiting of Thanatephorus cucumeris (Rhizoctonia solani) anastomosis group 1, subgroup IC. Mycologia 89:361–374
Julian MC, Acero J, Salazar O, Keijer J, Rubio V (1999) Mating typecorrelated molecular markers and demonstration of heterokaryosis in the phytopathogenic fungus Thanatephorus cucumeris (Rhizoctonia solani) AG 1-IC by AFLP DNA fingerprinting analysis. J Biotechnol 67:49–56
Ogoshi A (1972) On the perfect stage of anastomosis group 2 of Rhizoctonia solani Kuhn. Ann Phytopathol Soc Jpn 13:285–293
Ogoshi A (1976) Studies on the grouping of Rhizoctonia solani Kuhn with hyphal anastomosis, and on the perfect stage of groups. Bulletin Series C, No. 3. National Institute of Agricultural Science, Tokyo
Ogoshi A (1987) Ecology and pathogenicity of anastomosis and intraspecific groups of Rhizoctonia solani Kuhn. Annu Rev Phytopathol 25:125–143
Phillips AJL (1993) The use of protoplasts for the preparation of homokaryons from heterokaryotic isolates of Rhizoctonia solani. Mycol Res 97:456–460
Peberdy JF (1980) Protoplast fusion: a tool for genetic manipulation and breeding in industrial microorganisms. Enzyme Microb Technol 2:23–29
Sneh B, Burpee L, Ogoshi A (1991) Identification of Rhizoctonia species. American Phytopathological Society, St. Paul
Sneh B, Jabaji-Hare S, Neate S, Dijst G (1996) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Kluwer, Dordrecht
Stretton HM, Flentje NT, McKenzie AR (1967) Homothallism in Thanatephorus cucumeris. Aust J Biol Sci 20:103–112
Toda T, Hyakumachi M (2006) Heterokaryon formation in Thanatephorus cucumeris anastomosis group 2-2 IV. Mycologia 98:726–736
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA finger printing. Nucleic Acids Res 23:4407–4414
Whitney HS, Parmeter JR Jr (1963) Synthesis of heterokaryons in Rhizoctonia solani Kuhn. Can J Bot 41:879–886
Yang HA, Tommerup IC, Sivasithamparam K, O’Brien PA (1992) Heterokaryon formation with homokaryons derived from protoplasts of Rhizoctonia solani anastomosis group eight. Exp Mycol 16:268–278
Yoder OC (1988) Cochliobolus heterostrophus cause of southern corm leaf blight. In: Sidhu GS (ed) Advances in plant pathology, vol 6. Genetics of plant pathogenic fungi. Academic Press, London, pp 93–122
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Qu, P., Aratani, A., Syoji, T. et al. Use of single-protoplast isolates in the study of the mating phenomena of Rhizoctonia solani (Thanatephorus cucumeris) AG-1 IC and IA. Mycoscience 49, 132–137 (2008). https://doi.org/10.1007/s10267-007-0400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10267-007-0400-6