Abstract
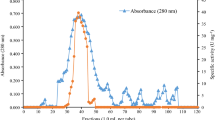
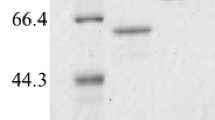
To investigate the function of amylases in the fruit-body formation of an ectomycorrhizal fungus, Lyophyllum shimeji, we purified the extracellular amylase in the medium of this fungus. The purified enzyme was obtained from 1.7 l stationary culture filtrate, with 4.2% recovery, and showed a single protein band on SDS-PAGE. The molecular mass was about 25 kDa. The enzyme was most active at around 40°C and pH 5.0 and stable over pH 4.5–6.5 for 30 min at 37°C. This amylase was remarkably activated by the presence of Ca2+ ion (7.7 times that of the control), but Ba2+ and Ag+ completely inhibited the activity. The amylase readily hydrolyzed the α-1,4 glucosidic linkage such as dextrin and amylose A (MW, 2900), converting into glucose, and hydrolyzed the α-1,6 glucosidic linkage of isomaltohexaose and amylopectin. However, the enzyme did not hydrolyze the cyclic polysaccharides. On the other hand, when a low molecular mass amylose A was hydrolyzed by this amylase, β-anomer glucose was produced. From these results, we concluded that the amylase from L. shimeji seems to be a glucoamylase.
Similar content being viewed by others
Author information
Authors and Affiliations
About this article
Cite this article
Kusuda, M., Ueda, M., Konishi, Y. et al. Characterization of extracellular glucoamylase from the ectomycorrhizal mushroom Lyophyllum shimeji . Mycoscience 45, 383–389 (2004). https://doi.org/10.1007/s10267-004-0196-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10267-004-0196-6