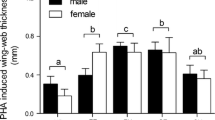
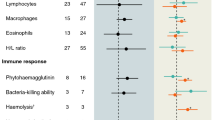
Abstract
Studies testing the “immunocompetence handicap hypothesis” have focussed on the immunosuppressive effects of androgens. Several recent studies have reported that mounting a humoral immune response might also result in a decrease in circulating androgen levels via a “negative feedback” on the hypothalamus–pituitary–gonadal axis (HPG). The aim of this correlative study was to analyse these immunosuppressive and HPG-suppressive interactions in reproductively active males of the peafowl. We collected blood samples of free living birds before and after challenging the immune system with a non-pathogenic antigen (sheep erythrocytes), and analysed immune parameters and plasma levels of the two main androgens in birds, testosterone and dihydrotestosterone. Males displaying larger versions of the main secondary sexual trait, the long and conspicuously ornamented train, tended to have higher androgen levels and significantly lower circulating levels of leukocytes, indicating that exaggerated ornaments might signal properties of the endocrine and immune system. Actual circulating levels of androgens did not correlate with the plasma levels of leukocytes and the antibody response to SRBC. However, changes in plasma levels of both androgens showed negative correlation with both leukocytes (P < 0.1) and SRBC responses (P < 0.05). The data therefore support the prediction that activity of the immune system is HPG-suppressive. Such suppression has been proposed to be especially costly during the reproductive season, during which androgens facilitate the expression of exaggerated traits that play an important role in sexual competition.

Similar content being viewed by others
References
Aitken ID, Parry SH (1974) The comparative serological response of the chicken, pheasant and quail to soluble and a particulate antigen. Immunology 27:623–629
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Apanius V (1998) Ontogeny of immunity in birds. In: Starck JM, Ricklefs RE (eds) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, New York, pp 203–222
Ball GF, Balthazart J (2004) Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav 83:329–346
Balthazart J, Schumacher M, Ottinger MA (1983) Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen Comp Endocrinol 51:191–207
Braude S, Tang-Martinez Z, Taylor GT (1999) Stress, testosterone, and the immunoredistribution hypothesis. Behav Ecol 10:345–350
Duckworth RA, Mendonca MT, Hill GE (2001) A condition dependent link between testosterone and disease resistance in the house finch. Proc R Soc Lond B 268:2467–2472
Fair JM, Hansen ES, Ricklefs RE (1999) Growth, developmental stability and immune responses in juvenile Japanese quails (Coturnix coturnix japonica). Proc R Soc Lond B 266:1735–1742
Folstad I, Karter A (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Fox A, Hudson PJ (2001) Parasites reduce territorial behaviour in red grouse (Lagopus lagopus scoticus). Ecol Lett 4:139–143
Garamszegi LZ, Møller AP, Torok J, Michl G, Peczely P, Richard M (2004) Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol 15:148–157
Getty T (2002) Signaling health versus parasites. Am Nat 159:363–371
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hart BL (1990) Behavioral adaptations to pathogens and parasites: five strategies. Neurosci Biobehav Rev 14:273–294
Hillgarth N, Wingfield JC (1997) Testosterone and immunosupression in vertebrates: implications for parasite-mediated sexual selection. In: Beckage NE (ed) Parasites and pathogens: effects on host hormones and behavior. Chapman and Hall, New York, pp 143–155
Hirschenhauser K, Oliveira RF (2006) Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav 71:265–277
Lister A, Van Der Kraak G (2002) Modulation of goldfish testicular testosterone production in vitro by tumor necrosis factor, interleukin-1, and macrophage conditioned media. J Exp Zool 292:477–486
Loyau A, Saint Jalme M, Cagniant C, Sorci G (2005a) Multiple sexual advertisements honestly reflect health status in peacocks (Pavo cristatus). Behav Ecol Sociobiol 58:552–557
Loyau A, Saint Jalme M, Sorci G (2005b) Intra- and intersexual selection for multiple traits in the peacock (Pavo cristatus). Ethology 111:810–820
Martin LBII, Scheuerlein A, Wikelski M (2003) Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond B 270:153–158
Møller AP, Petrie M (2002) Condition dependence, multiple sexual signals, and immunocompetence in peacocks. Behav Ecol 13:248–253
Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Horak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond B 268:1175–1181
Peters A, Delhey K, Denk AG, Kempenaers B (2004) Trade-offs between immune investment and sexual signaling in male mallards. Am Nat 164:51–59
Petrie M, Halliday T, Sanders C (1991) Peahens prefer peacocks with elaborate trains. Anim Behav 41:323–331
Poulin R (1995) “Adaptive” changes in the behaviour of parasitized animals: a critical review. Int J Parasitol 25:1371–1383
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68:227–239
Seto F, Henderson WG (1968) Natural and immune hemagglutinin forming capacity of immature chickens. J Exp Zool 16:501–512
Sheldon BC, Verhulst S (1996) Ecological immunity: costly parasite defences and trade-offs in evolutionary ecology. TREE 11:317–321
Snoeijs T, Eens M, Van-Den-Steen E, Pinxten R (2007) Kinetics of primary antibody responses to sheep red blood cells in birds: a literature review and new data from great tits and European starlings. Anim Biol 57:79–95
Svensson E, Råberg L, Koch C, Hasselquist D (1998) Energetic stress, immunosuppression and the costs of an antibody response. Funct Ecol 12:912–919
Weatherhead PJ, Metz KJ, Shutler D, Muma KE, Bennet GF (1995) Blood parasites and dominance in captive blackbirds. J Avian Biol 26:121–123
Weyts FAA, Cohen N, Flik G, Verburg-Van-Kemenade BML (1999) Interactions between the immune system and the hypothalamo–pituitary–interrenal axis in fish. Fish Shellfish Immunol 9:1–20
Wingfield JC, Farner DS (1975) The determination of five steroids in avian plasma by radioimmunoassay and competitive protein binding. Steroids 26:311–327
Wingfield JC, Hegner RE, Lewis D (1991) Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. J Zool Lond 225:43–58
Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990) The ‘‘challenge hypothesis’’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Yasmin SE, Yahya HSA (1996) Correlates of mating success in Indian peafowl. Auk 113:490–492
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–213
Acknowledgments
We thank the administration of the “Jardim da Estrela” in Lisbon and the “Parque Marechal Carmona” in Cascais for kindly helping us during the study. We are grateful to Lynn Erckmann for expert help with the hormone assays, and to Dr. Maria da Luz Ferreira and Dr. Eugénia Rebelo of LNIV of the Ministry of Agriculture, Portugal, for carrying out the immunological assays. We further thank the reviewers and Dr. Yoshitaka Tsubaki for their constructive comments on earlier versions of the manuscript. During this study AFHR was supported by a postdoctoral fellowship from the Portuguese Foundation for Science and Technology (FCT, SFRH/BPD/7143/2001). The research was further supported by FCT’s Plurianual Program to AFHR (R&D Unit 331/94), and a grant (IBN-9905679) from the National Science Foundation to JCW. The procedures used in this study comply with the Principles of Animal Care, publication No. 86–23, revised 1985, from the National Institutes of Health, USA, and with the current laws of Portugal.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ros, A.F.H., Correia, M., Wingfield, J.C. et al. Mounting an immune response correlates with decreased androgen levels in male peafowl, Pavo cristatus . J Ethol 27, 209–214 (2009). https://doi.org/10.1007/s10164-008-0105-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-008-0105-0