Abstract
Background
AI/ML CAD tools can potentially improve outcomes in the high-stakes, high-volume model of trauma radiology. No prior scoping review has been undertaken to comprehensively assess tools in this subspecialty.
Purpose
To map the evolution and current state of trauma radiology CAD tools along key dimensions of technology readiness.
Methods
Following a search of databases, abstract screening, and full-text document review, CAD tool maturity was charted using elements of data curation, performance validation, outcomes research, explainability, user acceptance, and funding patterns. Descriptive statistics were used to illustrate key trends.
Results
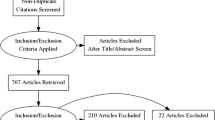
A total of 4052 records were screened, and 233 full-text articles were selected for content analysis. Twenty-one papers described FDA-approved commercial tools, and 212 reported algorithm prototypes. Works ranged from foundational research to multi-reader multi-case trials with heterogeneous external data. Scalable convolutional neural network–based implementations increased steeply after 2016 and were used in all commercial products; however, options for explainability were narrow. Of FDA-approved tools, 9/10 performed detection tasks. Dataset sizes ranged from < 100 to > 500,000 patients, and commercialization coincided with public dataset availability. Cross-sectional torso datasets were uniformly small. Data curation methods with ground truth labeling by independent readers were uncommon. No papers assessed user acceptance, and no method included human–computer interaction. The USA and China had the highest research output and frequency of research funding.
Conclusions
Trauma imaging CAD tools are likely to improve patient care but are currently in an early stage of maturity, with few FDA-approved products for a limited number of uses. The scarcity of high-quality annotated data remains a major barrier.



Similar content being viewed by others
References
Castro DC, Walker I, Glocker B (2020) Causality matters in medical imaging. Nat Commun 11(1):1–10
Willemink MJ, Koszek WA, Hardell C, Wu J, Fleischmann D, Harvey H, Folio LR, Summers RM, Rubin DL, Lungren MP (2020) Preparing medical imaging data for machine learning. Radiology 295(1):4–15
Waite S, Scott J, Gale B, Fuchs T, Kolla S, Reede D (2017) Interpretive error in radiology. Am J Roentgenol 208(4):739–749
Chung JH, Strigel RM, Chew AR, Albrecht E, Gunn ML (2009) Overnight resident interpretation of torso CT at a level 1 trauma center: an analysis and review of the literature. Acad Radiol 16(9):1155–1160
Bruno MA, Duncan JR, Bierhals AJ, Tappouni R (2018) Overnight resident versus 24-hour attending radiologist coverage in academic medical centers. Radiology 289(3):809–813
Banaste N, Caurier B, Bratan F, Bergerot J-F, Thomson V, Millet I (2018) Whole-body CT in patients with multiple traumas: factors leading to missed injury. Radiology 289(2):374–383
Glover M IV, Almeida RR, Schaefer PW, Lev MH, Mehan WA Jr (2017) Quantifying the impact of noninterpretive tasks on radiology report turn-around times. J Am Coll Radiol 14(11):1498–1503
Hunter TB, Taljanovic MS, Krupinski E, Ovitt T, Stubbs AY (2007) Academic radiologists’ on-call and late-evening duties. J Am Coll Radiol 4(10):716–719
Hanna TN, Loehfelm T, Khosa F, Rohatgi S, Johnson J-O (2016) Overnight shift work: factors contributing to diagnostic discrepancies. Emerg Radiol 23(1):41–47
Barquist ES, Pizano LR, Feuer W, Pappas PA, McKenney KA, LeBlang SD, Henry RP, Rivas LA, Cohn SM (2004) Inter-and intrarater reliability in computed axial tomographic grading of splenic injury: why so many grading scales? J Trauma Acute Care Surg 56(2):334–338
Clark R, Hird K, Misur P, Ramsay D, Mendelson R (2011) CT grading scales for splenic injury: why can’t we agree? J Med Imaging Radiat Oncol 55(2):163–169
Chen H, Unberath M, Dreizin D (2023) Toward automated interpretable AAST grading for blunt splenic injury. Emerg Radiol 30(1):41–50. https://doi.org/10.1007/s10140-022-02099-1
Furey AJ, O’Toole RV, Nascone JW, Sciadini MF, Copeland CE, Turen C (2009) Classification of pelvic fractures: analysis of inter-and intraobserver variability using the Young-Burgess and Tile classification systems. Orthopedics (Online) 32(6):401
Liu J, Varghese B, Taravat F, Eibschutz LS, Gholamrezanezhad A (2022) An extra set of intelligent eyes: application of artificial intelligence in imaging of abdominopelvic pathologies in emergency radiology. Diagnostics 12(6):1351
Krizhevsky A, Sutskever I, Hinton GE (2017) Imagenet classification with deep convolutional neural networks. Commun ACM 60(6):84–90
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition p. 770–778
Ronneberger O, Fischer P, Brox T (2015) U-Net: Convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A (eds) Medical image computing and computer-assisted intervention – MICCAI 2015. MICCAI 2015. Lecture notes in computer science(), vol 9351. Springer, Cham. https://doi.org/10.1007/978-3-319-24574-4_28
Zhou SK, Greenspan H, Davatzikos C, Duncan JS, Van Ginneken B, Madabhushi A, Prince JL, Rueckert D, Summers RM (2021) A review of deep learning in medical imaging: Imaging traits, technology trends, case studies with progress highlights, and future promises. Proc IEEE 109(5):820–838
Fujita H (2020) AI-based computer-aided diagnosis (AI-CAD): the latest review to read first. Radiol Phys Technol 13(1):6–19
West E, Mutasa S, Zhu Z, Ha R (2019) Global trend in artificial intelligence–based publications in radiology from 2000 to 2018. Am J Roentgenol 213(6):1204–1206
Harvey HB, Gowda V (2020) How the FDA regulates AI. Acad Radiol 27(1):58–61
Ebrahimian S, Kalra MK, Agarwal S, Bizzo BC, Elkholy M, Wald C, Allen B, Dreyer KJ (2022) FDA-regulated AI algorithms: trends, strengths, and gaps of validation studies. Acad Radiol 29(4):559–566
Sammer MB, Sher AC, Towbin AJ (2022) Ensuring adequate development and appropriate use of artificial intelligence in pediatric medical imaging. Am J Roentgenol 218(1):182–183
Yang L, Ene IC, Arabi Belaghi R, Koff D, Stein N, Santaguida PL (2022) Stakeholders' perspectives on the future of artificial intelligence in radiology: a scoping review. Eur Radiol 32(3):1477–1495. https://doi.org/10.1007/s00330-021-08214-z
Benjamens S, Dhunnoo P, Meskó B (2020) The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med 3(1):1–8
He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K (2019) The practical implementation of artificial intelligence technologies in medicine. Nat Med 25(1):30–36
Dreizin D, Munera F (2012) Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics 32(3):609–631. https://doi.org/10.1148/rg.323115099
Dreizin D, Munera F (2015) Multidetector CT for penetrating torso trauma: state of the art. Radiology 277(2):338–355
Varoquaux G, Cheplygina V (2022) Machine learning for medical imaging: methodological failures and recommendations for the future. NPJ Digit Med 5(1):1–8
Weikert T, Cyriac J, Yang S, Nesic I, Parmar V, Stieltjes B (2020) A practical guide to artificial intelligence–based image analysis in radiology. Invest Radiol 55(1):1–7
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol 8(1):19–32
Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA (2014) A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Meth 5(4):371–385
Langlotz CP, Allen B, Erickson BJ, Kalpathy-Cramer J, Bigelow K, Cook TS, Flanders AE, Lungren MP, Mendelson DS, Rudie JD (2019) A roadmap for foundational research on artificial intelligence in medical imaging: from the 2018 NIH/RSNA/ACR/The Academy Workshop. Radiology 291(3):781
Allen B Jr, Seltzer SE, Langlotz CP, Dreyer KP, Summers RM, Petrick N, Marinac-Dabic D, Cruz M, Alkasab TK, Hanisch RJ (2019) A road map for translational research on artificial intelligence in medical imaging: from the 2018 National Institutes of Health/RSNA/ACR/The Academy Workshop. J Am Coll Radiol 16(9):1179–1189
Majkowska A, Mittal S, Steiner DF, Reicher JJ, McKinney SM, Duggan GE, Eswaran K, Cameron Chen P-H, Liu Y, Kalidindi SR (2020) Chest radiograph interpretation with deep learning models: assessment with radiologist-adjudicated reference standards and population-adjusted evaluation. Radiology 294(2):421–431
Seah JC, Tang CH, Buchlak QD, Holt XG, Wardman JB, Aimoldin A, Esmaili N, Ahmad H, Pham H, Lambert JF (2021) Effect of a comprehensive deep-learning model on the accuracy of chest x-ray interpretation by radiologists: a retrospective, multireader multicase study. Lancet Digit Health 3(8):e496–e506
Jones RM, Sharma A, Hotchkiss R, Sperling JW, Hamburger J, Ledig C, O’Toole R, Gardner M, Venkatesh S, Roberts MM (2020) Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs. NPJ Digit Med 3(1):1–6
Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, Mahajan V, Rao P, Warier P (2018) Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet 392(10162):2388–2396
Dreizin D, Zhou Y, Chen T, Li G, Yuille AL, McLenithan A, Morrison JJ (2020) Deep learning-based quantitative visualization and measurement of extraperitoneal hematoma volumes in patients with pelvic fractures: potential role in personalized forecasting and decision support. J Trauma Acute Care Surg 88(3):425
Harris RJ, Kim S, Lohr J, Towey S, Velichkovich Z, Kabachenko T, Driscoll I, Baker B (2019) Classification of aortic dissection and rupture on post-contrast CT images using a convolutional neural network. J Digit Imaging 32(6):939–946
Ginat DT (2020) Analysis of head CT scans flagged by deep learning software for acute intracranial hemorrhage. Neuroradiology 62(3):335–340
Ginat D (2021) Implementation of machine learning software on the radiology worklist decreases scan view delay for the detection of intracranial hemorrhage on CT. Brain Sci 11(7):832
Kundisch A, Hönning A, Mutze S, Kreissl L, Spohn F, Lemcke J, Sitz M, Sparenberg P, Goelz L (2021) Deep learning algorithm in detecting intracranial hemorrhages on emergency computed tomographies. PLoS ONE 16(11):e0260560
Ojeda P, Zawaideh M, Mossa-Basha M, Haynor D (2019) The ional neural network for detection of intracranial bleeds on non-contrast head computed tomography studies. In: Proc. SPIE 10949, Medical Imaging 2019: Image processing, 109493J. https://doi.org/10.1117/12.2513167
Kau T, Ziurlys M, Taschwer M, Kloss-Brandstätter A, Grabner G, Deutschmann H (2022) FDA-approved deep learning software application versus radiologists with different levels of expertise: detection of intracranial hemorrhage in a retrospective single-center study. Neuroradiology 64(5):981–990
Voter AF, Meram E, Garrett JW, John-Paul JY (2021) Diagnostic accuracy and failure mode analysis of a deep learning algorithm for the detection of intracranial hemorrhage. J Am Coll Radiol 18(8):1143–1152
Wismüller A, Stockmaster L (2020) A prospective randomized clinical trial for measuring radiology study reporting time on artificial intelligence-based detection of intracranial hemorrhage in emergent care head CT. In: Proc. SPIE 11317, Medical Imaging 2020: Biomedical applications in molecular, structural, and functional imaging, 113170M. https://doi.org/10.1117/12.2552400
Heit J, Coelho H, Lima F, Granja M, Aghaebrahim A, Hanel R, Kwok K, Haerian H, Cereda C, Venkatasubramanian C (2021) Automated cerebral hemorrhage detection using RAPID. Am J Neuroradiol 42(2):273–278
Gipson J, Tang V, Seah J, Kavnoudias H, Zia A, Lee R, Mitra B, Clements W (2022) Diagnostic accuracy of a commercially available deep-learning algorithm in supine chest radiographs following trauma. Br J Radiol 95:20210979
Small J, Osler P, Paul A, Kunst M (2021) Ct cervical spine fracture detection using a convolutional neural network. Am J Neuroradiol 42(7):1341–1347
Voter A, Larson M, Garrett J, Yu J-P (2021) Diagnostic accuracy and failure mode analysis of a deep learning algorithm for the detection of cervical spine fractures. Am J Neuroradiol 42(8):1550–1556
Weikert T, Noordtzij LA, Bremerich J, Stieltjes B, Parmar V, Cyriac J, Sommer G, Sauter AW (2020) Assessment of a deep learning algorithm for the detection of rib fractures on whole-body trauma computed tomography. Korean J Radiol 21(7):891
Hayashi D, Kompel AJ, Ventre J, Ducarouge A, Nguyen T, Regnard N-E, Guermazi A (n.d.) Automated detection of acute appendicular skeletal fractures in pediatric patients using deep learning. Skelet Radiol 2022:1–11
Hayashi D, Kompel AJ, Ventre J, Ducarouge A, Nguyen T, Regnard NE, Guermazi A (2022) Automated detection of acute appendicular skeletal fractures in pediatric patients using deep learning. Skeletal Radiol 51(11):2129–2139. https://doi.org/10.1007/s00256-022-04070-0
Duron L, Ducarouge A, Gillibert A, Lainé J, Allouche C, Cherel N, Zhang Z, Nitche N, Lacave E, Pourchot A (2021) Assessment of an AI aid in detection of adult appendicular skeletal fractures by emergency physicians and radiologists: a multicenter cross-sectional diagnostic study. Radiology 300(1):120–129
Dupuis M, Delbos L, Veil R, Adamsbaum C (2022) External validation of a commercially available deep learning algorithm for fracture detection in children. Diagn Interv Imaging 103(3):151–159
Rueckel J, Sperl JI, Kaestle S, Hoppe BF, Fink N, Rudolph J, Schwarze V, Geyer T, Strobl FF, Ricke J (2021) Reduction of missed thoracic findings in emergency whole-body computed tomography using artificial intelligence assistance. Quant Imaging Med Surg 11:2486–2498
Genant HK, Li J, Wu CY, Shepherd JA (2000) Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom 3(3):281–290
Davis MA, Rao B, Cedeno PA, Saha A, Zohrabian VM (2022) Machine learning and improved quality metrics in acute intracranial hemorrhage by noncontrast computed tomography. Curr Probl Diagn Radiol 51(4):556–561. https://doi.org/10.1067/j.cpradiol.2020.10.007
Shin H-C et al (2016) Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging 35(5):1285–1298. https://doi.org/10.1109/TMI.2016.2528162
Remedios SW, Roy S, Bermudez C, Patel MB, Butman JA, Landman BA, Pham DL (2020) Distributed deep learning across multisite datasets for generalized CT hemorrhage segmentation. Med Phys 47(1):89–98
Mutasa S, Varada S, Goel A, Wong TT, Rasiej MJ (2020) Advanced deep learning techniques applied to automated femoral neck fracture detection and classification. J Digit Imaging 33(5):1209–1217
Zhou Y, Dreizin D, Wang Y, Liu F, Shen W, Yuille AL (2021) External attention assisted multi-phase splenic vascular injury segmentation with limited data. IEEE Trans Med Imaging 41(6):1346–1357
Lind A, Akbarian E, Olsson S, Nåsell H, Sköldenberg O, Razavian AS, Gordon M (2021) Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system. PLoS ONE 16(4):e0248809
Jin L, Yang J, Kuang K, Ni B, Gao Y, Sun Y, Gao P, Ma W, Tan M, Kang H (2020) Deep-learning-assisted detection and segmentation of rib fractures from CT scans: Development and validation of FracNet. EBioMedicine 62:103106
Zhou Q-Q, Hu Z-C, Tang W, Xia Z-Y, Wang J, Zhang R, Li X, Chen C-Y, Zhang B, Lu L (2022) Precise anatomical localization and classification of rib fractures on CT using a convolutional neural network. Clin Imaging 81:24–32
Olczak J, Emilson F, Razavian A, Antonsson T, Stark A, Gordon M (2020) Ankle fracture classification using deep learning: automating detailed AO Foundation/Orthopedic Trauma Association (AO/OTA) 2018 malleolar fracture identification reaches a high degree of correct classification. Acta Orthop 92(1):102–108
Huang Y-J, Liu W, Wang X, Fang Q, Wang R, Wang Y, Chen H, Chen H, Meng D, Wang L (2020) Rectifying supporting regions with mixed and active supervision for rib fracture recognition. IEEE Trans Med Imaging 39(12):3843–3854
Luo J, Kitamura G, Doganay E, Arefan D, Wu S (2021) Medical knowledge-guided deep curriculum learning for elbow fracture diagnosis from x-ray images. In: Proc. SPIE 11597, Medical Imaging 2021: Computer-aided diagnosis, 1159712. https://doi.org/10.1117/12.2582184
Zapaishchykova A, Dreizin D, Li Z, Wu JY, Faghihroohi S, Unberath M (2021) An interpretable approach to automated severity scoring in pelvic trauma. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2021. MICCAI 2021, vol 12903. Lecture Notes in Computer Science(), Springer, Cham. https://doi.org/10.1007/978-3-030-87199-4_40
Diaz-Pinto A, Alle S, Ihsani A, Asad M, Nath V, Pérez-García F, Mehta P, Li W, Roth HR, Vercauteren T (2022) Monai label: a framework for ai-assisted interactive labeling of 3d medical images. arXiv preprint arXiv:220312362
Diaz-Pinto A, Mehta P, Alle S, Asad M, Brown R, Nath V, Ihsani A, Antonelli M, Palkovics D, Pinter C (2022) DeepEdit: deep editable learning for interactive segmentation of 3D medical images. MICCAI Workshop on Data Augmentation, Labelling, and Imperfections: Springer, p. 11–21
Burns JE, Yao J, Muñoz H, Summers RM (2016) Automated detection, localization, and classification of traumatic vertebral body fractures in the thoracic and lumbar spine at CT. Radiology 278(1):64
Bandyopadhyay O, Biswas A, Bhattacharya BB (2016) Long-bone fracture detection in digital X-ray images based on digital-geometric techniques. Comput Methods Programs Biomed 123:2–14
Sun L, Kong Q, Huang Y, Yang J, Wang S, Zou R, Yin Y, Peng J (2020) Automatic segmentation and measurement on knee computerized tomography images for patellar dislocation diagnosis. Comput Math Methods Med 2020
Seo JW, Lim SH, Jeong JG, Kim YJ, Kim KG, Jeon JY (2021) A deep learning algorithm for automated measurement of vertebral body compression from X-ray images. Sci Rep 11(1):1–10
Baum T, Bauer JS, Klinder T, Dobritz M, Rummeny EJ, Noël PB, Lorenz C (2014) Automatic detection of osteoporotic vertebral fractures in routine thoracic and abdominal MDCT. Eur Radiol 24(4):872–880
Xia X, Zhang X, Huang Z, Ren Q, Li H, Li Y, Liang K, Wang H, Han K, Meng X (2021) Automated detection of 3D midline shift in spontaneous supratentorial intracerebral haemorrhage with non-contrast computed tomography using deep convolutional neural networks. Am J Transl Res 13(10):11513
Guo J, Mu Y, Xue D, Li H, Chen J, Yan H, Xu H, Wang W (2021) Automatic analysis system of calcaneus radiograph: Rotation-invariant landmark detection for calcaneal angle measurement, fracture identification and fracture region segmentation. Comput Methods Programs Biomed 206:106124
Monchka BA, Kimelman D, Lix LM, Leslie WD (2021) Feasibility of a generalized convolutional neural network for automated identification of vertebral compression fractures: the Manitoba Bone Mineral Density Registry. Bone 150:116017
Rajpurkar P, Irvin J, Bagul A, Ding D, Duan T, Mehta H, Yang B, Zhu K, Laird D, Ball RL (2017) Mura: Large dataset for abnormality detection in musculoskeletal radiographs. arXiv preprint arXiv:171206957
Wang Y, Wang K, Peng X, Shi L, Sun J, Zheng S, Shan F, Shi W, Liu L (2021) DeepSDM: Boundary-aware pneumothorax segmentation in chest X-ray images. Neurocomputing 454:201–211
Flanders AE, Prevedello LM, Shih G, Halabi SS, Kalpathy-Cramer J, Ball R, Mongan JT, Stein A, Kitamura FC, Lungren MP (2020) Construction of a machine learning dataset through collaboration: the RSNA 2019 brain CT hemorrhage challenge. Radiology: Artif Intell 2(3):e190211
Wang X, Peng Y, Lu L, Lu Z, Bagheri M, Summers RM (2017) Chestx-ray8: Hospital-scale chest X-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. Proceedings of the IEEE conference on computer vision and pattern recognition, p. 2097–2106
Choi JW, Cho YJ, Ha JY, Lee YY, Koh SY, Seo JY, Choi YH, Cheon J-E, Phi JH, Kim I (2022) Deep learning-assisted diagnosis of pediatric skull fractures on plain radiographs. Korean J Radiol 23(3):343
Oakden-Rayner L, Gale W, Bonham TA, Lungren MP, Carneiro G, Bradley AP, Palmer LJ (2022) Validation and algorithmic audit of a deep learning system for the detection of proximal femoral fractures in patients in the emergency department: a diagnostic accuracy study. Lancet Digit Health 4(5):e351–e358
Jiménez-Sánchez A, Kazi A, Albarqouni S, Kirchhoff C, Biberthaler P, Navab N, Kirchhoff S, Mateus D (2020) Precise proximal femur fracture classification for interactive training and surgical planning. Int J Comput Assist Radiol Surg 15(5):847–857
Derkatch S, Kirby C, Kimelman D, Jozani MJ, Davidson JM, Leslie WD (2019) Identification of vertebral fractures by convolutional neural networks to predict nonvertebral and hip fractures: a registry-based cohort study of dual X-ray absorptiometry. Radiology 293(2):405–411
Cai W, Lee J-G, Fikry K, Yoshida H, Novelline R, de Moya M (2012) MDCT quantification is the dominant parameter in decision-making regarding chest tube drainage for stable patients with traumatic pneumothorax. Comput Med Imaging Graph 36(5):375–386
Dreizin D, Zhou Y, Fu S, Wang Y, Li G, Champ K, Siegel E, Wang Z, Chen T, Yuille AL (2020) A multiscale deep learning method for quantitative visualization of traumatic hemoperitoneum at CT: Assessment of feasibility and comparison with subjective categorical estimation. Radiol Artif Intell 2(6):e190220. https://doi.org/10.1148/ryai.2020190220
Dreizin D, Goldmann F, LeBedis C, Boscak A, Dattwyler M, Bodanapally U, Li G, Anderson S, Maier A, Unberath M (2021) An automated deep learning method for tile AO/OTA pelvic fracture severity grading from trauma whole-body CT. J Digit Imaging 34(1):53–65
Dreizin D, Chen T, Liang Y, Zhou Y, Paes F, Wang Y, Yuille AL, Roth P, Champ K, Li G (2021) Added value of deep learning-based liver parenchymal CT volumetry for predicting major arterial injury after blunt hepatic trauma: a decision tree analysis. Abdom Radiol 46(6):2556–2566
Okimatsu S, Maki S, Furuya T, Fujiyoshi T, Kitamura M, Inada T, Aramomi M, Yamauchi T, Miyamoto T, Inoue T (2022) Determining the short-term neurological prognosis for acute cervical spinal cord injury using machine learning. J Clin Neurosci 96:74–79
McCoy D, Dupont S, Gros C, Cohen-Adad J, Huie R, Ferguson A, Duong-Fernandez X, Thomas L, Singh V, Narvid J (2019) Convolutional neural network–based automated segmentation of the spinal cord and contusion injury: deep learning biomarker correlates of motor impairment in acute spinal cord injury. Am J Neuroradiol 40(4):737–744
Chaganti S, Plassard AJ, Wilson L, Smith MA, Patel MB, Landman BA (2016) A Bayesian framework for early risk prediction in traumatic brain injury. Proc SPIE Int Soc Opt Eng 27(9784):978422. https://doi.org/10.1117/12.2217306
Cai Y, Wu S, Zhao W, Li Z, Wu Z, Ji S (2018) Concussion classification via deep learning using whole-brain white matter fiber strains. PLoS ONE 13(5):e0197992
Hellyer PJ, Leech R, Ham TE, Bonnelle V, Sharp DJ (2013) Individual prediction of white matter injury following traumatic brain injury. Ann Neurol 73(4):489–499
Kim Y-T, Kim H, Lee C-H, Yoon BC, Kim JB, Choi YH, Cho W-S, Oh B-M, Kim D-J (2021) Intracranial densitometry-augmented machine learning enhances the prognostic value of brain CT in pediatric patients with traumatic brain injury: A retrospective pilot study. Front Pediatr 9:750272. https://doi.org/10.3389/fped.2021.750272
Mohamed M, Alamri A, Mohamed M, Khalid N, O'Halloran P, Staartjes V, Uff C (2022) Prognosticating outcome using magnetic resonance imaging in patients with moderate to severe traumatic brain injury: A machine learning approach. Brain Inj 36(3):353–358. https://doi.org/10.1080/02699052.2022.2034184
Yao H, Williamson C, Gryak J, Najarian K (2020) Automated hematoma segmentation and outcome prediction for patients with traumatic brain injury. Artif Intell Med 107:101910
Choi J, Mavrommati K, Li NY, Patil A, Chen K, Hindin DI, Forrester JD (2022) Scalable deep learning algorithm to compute percent pulmonary contusion among patients with rib fractures. J Trauma Acute Care Surg 93(4):461–466
Röhrich S, Hofmanninger J, Negrin L, Langs G, Prosch H (2021) Radiomics score predicts acute respiratory distress syndrome based on the initial CT scan after trauma. Eur Radiol 31(8):5443–5453
Lee S, Summers RM (2021) Clinical artificial intelligence applications in radiology: chest and abdomen. Radiol Clin 59(6):987–1002
Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL (2020) Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT. J Digit Imaging 33(1):243–251
Chen H, Gomez C, Huang C-M, Unberath M (2022) Explainable medical imaging AI needs human-centered design: guidelines and evidence from a systematic review. npj Digit Med 5(1):1–15
Mongan J, Moy L, Kahn CE Jr (2020) Checklist for Artificial Intelligence in Medical Imaging (CLAIM): A guide for authors and reviewers. Radiol Artif Intell 2(2):e200029. https://doi.org/10.1148/ryai.2020200029
Sounderajah V, Ashrafian H, Aggarwal R, De Fauw J, Denniston AK, Greaves F, Karthikesalingam A, King D, Liu X, Markar SR (2020) Developing specific reporting guidelines for diagnostic accuracy studies assessing AI interventions: the STARD-AI Steering Group. Nat Med 26(6):807–808
Sounderajah V, Ashrafian H, Golub RM, Shetty S, De Fauw J, Hooft L, Moons K, Collins G, Moher D, Bossuyt PM (2021) Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: the STARD-AI protocol. BMJ Open 11(6):e047709
Funding
David Dreizin funding source: NIH K08 EB027141-01A1 (PI: David Dreizin, MD)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dreizin, D., Staziaki, P.V., Khatri, G.D. et al. Artificial intelligence CAD tools in trauma imaging: a scoping review from the American Society of Emergency Radiology (ASER) AI/ML Expert Panel. Emerg Radiol 30, 251–265 (2023). https://doi.org/10.1007/s10140-023-02120-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-023-02120-1