Abstract
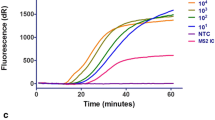
The aim of the present study was to compare traditional methods for the detection of respiratory syncytial virus with a newly developed commercial assay based on real-time nucleic acid sequence based amplification. Respiratory syncytial virus is a major cause of severe respiratory infection in infants and in certain groups of older children and adults. Treatment options are limited, but a rapid diagnosis improves patient management and infection control. The rapid diagnosis of respiratory syncytial virus currently relies on antigen detection assays. These tests are limited to use in certain good-quality types of samples, which are rarely obtained from adult patients. Molecular-based assays for the detection of respiratory syncytial virus are shown to be highly sensitive, specific, and more rapid than cell culture techniques. This retrospective study compared traditional laboratory techniques for the detection of respiratory syncytial virus in 508 respiratory samples collected during the winter months of 2003–2004 against the recently developed, commercially available NucliSens EasyQ Respiratory Syncytial Virus A+B assay (bioMérieux, Marcy l’Etoile, France), which is based on real-time nucleic acid sequence based amplification using molecular beacons and an internal control. Using traditional techniques, the prevalence of respiratory syncytial virus in the samples tested was found to be 21%. Using the real-time nucleic acid sequence-based amplification assay, an additional 41 samples from patients with a clinically diagnosed respiratory illness were found to be positive for respiratory syncytial virus. The NucliSens EasyQ assay was shown to be sensitive and specific for the detection of respiratory syncytial virus A+B in different types of respiratory samples. Moreover, the time required to complete the assay was <4 h, so results could be obtained on the same day as sample receipt in the laboratory.

Similar content being viewed by others
References
Damachowske JB, Rosenberg HF (1999) Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 12:298–309
Falsey AR, Walsh E (2000) Respiratory syncytial virus infection in adults. Clin Microbiol Rev 13:371–384
Welliver RC (2004) Respiratory syncytial virus infection: therapy and prevention. Paediatr Resp Rev 5(Suppl A):S127–S133
Dudas RA, Karron RA (1998) Respiratory syncytial virus vaccines. Clin Microbiol Rev 11:430–439
Walsh EE, McConnochie KM, Long CE, Hall CB (1997) Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis 175:814–820
Sullender WM (2000) Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev 13:1–15
Kafetzis DA (2004) Prophylaxis, therapy and prevention of viral respiratory infections. Paediatr Resp Rev 5(Suppl A):S185–S189
Englund JA, Piedra P, Jewell A, Baxter BB, Whimbey E, Patel K (1996) Rapid diagnosis of respiratory syncytial virus in immunocompromised adults. J Clin Microbiol 34:1649–1653
Hall CB, Douglas RG, Gieman JM (1976) Respiratory syncytial virus infection in infants: quantitation and duration of shedding. J Pediatr 131:1–5
Falsey AR, Formica MA, Walsh EE (2002) Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 40:817–820
Henrickson KJ (2004) Advances in the laboratory diagnosis of viral respiratory disease. Pediatr Infect Dis J 23:S6–S10
Simoes EAF (2001) Overlap between respiratory syncytial virus infection and influenza. Commentary. Lancet 358:1382–1383
Paton AW, Paton JC, Lawrence AJ, Goldwater PN, Harris RJ (1992) Rapid detection of respiratory syncytial virus in nasopharyngeal aspirates by reverse transcription and polymerase chain reaction amplification. J Clin Microbiol 30:901–904
Hu A, Colella M, Tam JS, Rappaport R, Cheng SM (2003) Simultaneous detection, subgrouping and quantitation of respiratory syncytial virus A and B by real-time PCR. J Clin Microbial 41:149–154
Borg I, Rohde G, Löseke S, Bittscheidt J, Schultze-Weninghaus G, Stephan V, Bufe A (2003) Evaluation of a quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J 21:944–951
Boivin G, Côté S, Déry P, De Serres G, Bergeron MG (2004) Multiplex real-time PCR assay for detection of influenza and human respiratory syncytial viruses. J Clin Microbiol 42:45–51
Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ACJ (2004) Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3 and 4. J Clin Microbiol 2042:1564–1569
Dingle KE, Crook D, Jeffery K (2004) Stable and noncompetitive RNA internal control for routine clinical diagnostic reverse transcription-PCR. J Clin Microbiol 42:1003–1011
Deiman B, Van Aarle P, Sillekens P (2002) Characteristics and applications of nucleic acid sequence-based amplification (NASBA). Mol Biotech 20:163–179
Fox JD, Song H, Samuelson A, Zhang Y, Neale ML, Westmoreland D (2002) Development and evaluation of nucleic acid sequence based amplification (NASBA) for the diagnosis of Enterovirus infections using the Nuclisen basic kit. J Clin Virol 24:117–130
Hibbitts S, Fox JD (2004) Real-time NASBA. In: Saunders N, Logan J, Edwards K (eds) Methods in real-time PCR. Horizon Scientific, Hethersett, UK, pp 225–241
Hibbitts S, Rahman A, John R, Westmoreland D, Fox JD (2003) Development and evaluation of NucliSens Basic Kit NASBA for diagnosis of parainfluenza virus infection. J Virol Methods 108:145–155
Hibbitts S, Fox JD (2002) The application of molecular techniques to diagnosis of viral respiratory tract infections. Rev Med Microbiol 13:177–185
Moore C, Hibbitts S, Owen N, Corden S, Harrison G, Fox J, Gelder C, Westmoreland D (2004) Development and evaluation of a real-time nucleic acid sequence based amplification assay for rapid detection of influenza A. J Med Virol 74:619–628
Boom R, Sol CJ, Salimans MMM, Hansen CL, Wertheim-van Dillen PME, Noordaa van de J (1990) Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503
Deiman B, Jacobs F, Schrover C, Vermeer S, van de Wiel P (2004) Development of NucliSens EasyQ Respiratory Syncytial Virus reagents. In: Program and abstracts of the 2nd Eurovirology Meeting, Madrid, 5–9 September 2005, Abstract no. P10 17
Stockton J, Ellis JS, Saville M, Clewley JP, Zambon MC (1998) Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. J Clin Microbiol 36:2990–2995
Newcombe RG (1998) Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17:2635–2650
Acknowledgements
We would like to thank the staff of the Wales Specialist Virologist Centre and other hospital laboratories in Wales who performed the traditional laboratory assays. Their contribution was instrumental to the completion of this study. Technical support and the reagents for the real-time NASBA assay were provided by bioMérieux.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, C., Valappil, M., Corden, S. et al. Enhanced clinical utility of the NucliSens EasyQ RSV A+B Assay for rapid detection of respiratory syncytial virus in clinical samples. Eur J Clin Microbiol Infect Dis 25, 167–174 (2006). https://doi.org/10.1007/S10096-006-0112-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/S10096-006-0112-4