Abstract
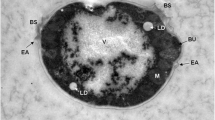
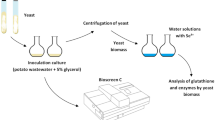
The work deals with lipid modifications of pigment-forming yeasts Rhodotorula and Sporobolomyces growing under presence of selenium. This metal in the medium significantly prolonged lag-phase of all cultures and enlarged yeast cells. Total, neutral, and membrane yeast lipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol) consisted of predominantly palmitic, palmitoleic, stearic, oleic, linoleic, and linolenic acids. Selenium activated fatty acid unsaturation mainly in phosphatidylcholine due to elevated levels of linoleic and linolenic acids. Because biosynthesis of C18 unsaturated fatty acids in Rhodotorula and Sporobolomyces species may be associated with phosphatidylcholine moieties, selenium might be involved to the induction of membranebound fatty acid Δ12 and Δ15 desaturases in red yeasts. Oppositely, neutral lipids (primarily triacylglycerols) did not show such intensive changes in fatty acid composition as their polar counterparts. These observations could be applied for preparation of selenized red yeasts containing carotenoid pigments with enhanced accumulation of linoleic and linolenic acids.
Similar content being viewed by others
References
Navarro-Alarcon M, Cabera-Vique C. Selenium in food and the human body: A review. Sci. Total Environ. 400: 115–141 (2008)
Tapiero H, Townsend DM, Tew KD. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 57: 134–144 (2003)
Wood SM, Beckham C, Yosioka A, Darban H, Watson RR. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. 2: 85–92 (1999)
Rezanka T, Sigler K. Biologically active compounds of semi-metals. Phytochemistry 69: 585–606 (2008)
Pedrero Z, Madrid Y. Novel approaches for selenium speciation in foodstuffs, and biological speciments: A review. Anal. Chim. Acta 634: 135–152 (2009)
Danch A, Chmielowski J. Selenium bio-accumulation in Saccharomyces cerevisiae cells. Acta Biol. Siles. 18: 57–64 (1985)
Pankiewicz U, Jamroz J, Schodzinski A. Optimization of selenium accumulation in Rhodotorula rubra cells by treatment of culturing medium with pulse electric field. Int. Agrophys. 20: 147–152 (2006)
Yin H, Chen Z, Gu Z, Han Y. Optimization of natural fermentative medium for selenium-enriched yeast by D-optimal mixture design. LWT-Food Sci. Technol. 42: 327–331 (2009)
Encinar JR, Sliwka-Kaszynska M, Polatajko A, Vacchina V, Szpunar J. Methodological advances for selenium speciation analysis in yeast. Anal. Chim. Acta 500: 171–183 (2003)
Stabnikova O, Ivanov V, Larionova I, Stabnikov V, Bryszewska MA, Lewis J. Ukrainian dietary bakery product with seleniumenriched yeast. LWT-Food Sci. Technol. 41: 890–895 (2008)
Wang Y-B, Xu B-H. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim. Feed Sci. Tech. 144: 306–314 (2008)
Juniper DT, Phipps RH, Ramos-Morales EG, Bertin G. Effect of high dose selenium enriched yeast diets on the distribution of total selenium and selenium species within lamb tissues. Livest. Sci. 122: 63–67 (2009)
Czauderna M, Kowalczyk J, Korniluk K. Effect of dietary conjugated linoleic acid and selenized yeast on the concentration of fatty acids and minerals in rats. Arch. Anim. Nutr. 61: 135–150 (2007)
Breierová E, Gregor T, Márová I, Čertík M, Kogan G. Enhanced antioxidant formula based on a selenium-supplemented carotenoid producing yeast biomass. Chem. Biodivers. 5: 440–446 (2008)
Čertík M, Hanusová V, Breierová E, Márová I, Rapta P. Biotechnological production and properties of carotenoid pigments. pp. 355–375. In: Biocatalysis and Agricultural Biotechnology. Hou CT, Shaw J-F (eds). CRC Press, Inc., Boca Raton, FL, USA (2009)
Breierová E, Čertík M, Kovárová A, Gregor T. Effect of nickel on the yeasts in the osmotic unsuitable environment. Z. Naturforsch. 63: 873–878 (2008)
Čertík M, Breierová E, Juršíková P. Effect of cadmium on lipid composition of Aureobasidium pullulans grown under addition of extracellular polysaccharides. Int. Biodeter. Biodegr. 55: 195–202 (2005)
Čertík M, Andráši P, Šajbidor J. Effect of extraction methods on lipid yield and fatty acid composition of lipid classes containing γ-linolenic acid extracted from fungi. J. Am. Oil Chem. Soc. 73: 357–365 (1996)
Čertík M, Shimizu S. Kinetic analysis of oil biosynthesis by arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl. Microbiol. Biot. 54: 224–230 (2000)
Christoperson SW, Glass RL. Preparation of milk fat methyl esters by alcoholysis in an essentially nonalcoholic solution. J. Dairy Sci. 52: 1289–1290 (1969)
Čertík M, Sakuradani E, Shimizu S. Desaturase-defective fungal mutants: Useful tools for the regulation and overproduction of polyunsaturated fatty acids. Trends Biotechnol. 16: 500–505 (1998)
Kates M, Pugh EL, Ferrante G. Regulation of membrane fluidity by lipid desaturases. pp. 379–395. In: Membrane Fluidity. Kates M, Manson LA (eds). Plenum Publ. Co., New York, NY, USA (1984)
Jackson FM, Fraser TCM, Smith MA, Lazarus C, Stobart AK, Griffiths G. Biosynthesis of C18 polyunsaturated fatty acids in microsomal membrane preparations from the filamentous fungus Mucor circinelloides. Eur. J. Biochem. 252: 513–519 (1998)
Yu LL, Wang RL, Zhang YZ, Kleemann DO, Zhu XP, Jia ZH. Effects of selenium supplementation on polyunsaturated fatty acid concentrations and antioxidant status in plasma and liver of lambs fed linseed oil or sunflower oil diets. Anim. Feed Sci. Techn. 140: 39–51 (2008)
Schäfer K, Kyriakopoulos A, Gessner H, Grune T, Behne D. Effects of selenium deficiency on fatty acid metabolism in rats fed fish oilenriched diets. J. Trace Elem. Med. Biol. 14: 89–97 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Čertík, M., Breierová, E., Oláhová, M. et al. Effect of selenium on lipid alternations in pigment-forming yeasts. Food Sci Biotechnol 22 (Suppl 1), 45–51 (2013). https://doi.org/10.1007/s10068-013-0047-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-013-0047-3