Abstract
Circulating endothelial progenitor cells (EPCs) contribute to vascular repair and their monitoring could have prognostic clinical value. Exercise is often prescribed for the management of cardiometabolic diseases, however, it is not fully understood how it regulates EPCs. Objectives: to systematically examine the acute and chronic effects of different exercise modalities on circulating EPCs in patients with cardiovascular and metabolic disease. Methods: Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. Results: six electronic databases and reference lists of eligible studies were searched to April 2021. Thirty-six trials met the inclusion criteria including 1731 participants. Acute trials: in chronic heart failure (CHF), EPC mobilisation was acutely increased after high intensity interval or moderate intensity continuous exercise training, while findings were inconclusive after a cardiopulmonary cycling exercise test. Maximal exercise tests acutely increased EPCs in ischaemic or revascularized coronary artery disease (CAD) patients. In peripheral arterial disease (PAD), EPC levels increased up to 24 h post-exercise. In patients with compromised metabolic health, EPC mobilisation was blunted after a single exercise session. Chronic trials: in CHF and acute coronary syndrome, moderate intensity continuous protocols, with or without resistance exercise or calisthenics, increased EPCs irrespective of EPC identification phenotype. Findings were equivocal in CAD regardless of exercise mode, while in severe PAD disease EPCs increased. High intensity interval training increased EPCs in hypertensive metabolic syndrome and heart failure reduced ejection fraction. Conclusion: the clinical condition and exercise modality influence the degree of EPC mobilisation and magnitude of EPC increases in the long term.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vascular endothelium has many important and diverse functions (Hadi et al. 2005) with regulation of vascular homeostasis being one of the most important ones (Verma and Anderson 2002). Endothelial dysfunction, which is an independent predictor of cardiovascular events, precedes the development of atherosclerosis and cardiovascular disease (CVD) (Widlansky et al. 2003; Gokce et al. 2003). Progression of diseases such as metabolic syndrome and diabetes mellitus (DM) is also strongly associated with the progression of endothelial dysfunction (Fornoni and Raij 2005; Hadi and Suwaidi 2007). At the same time, several cardiovascular risk factors such as ageing, physical inactivity, smoking and hypertension contribute to an exacerbation of endothelial dysfunction as demonstrated by reduced flow-mediated dilatation (FMD) (Steinberg et al. 1996; Black et al. 2009; Amato et al. 2013). Therefore, endothelial repair is essential for the healing of injured endothelium and the prevention of endothelial dysfunction.
Mature endothelial cells have low proliferative capacity (Hristov et al. 2003; Werner and Nickenig 2006) and it was once thought that vasculogenesis, occurred only during embryonic development (Koutroumpi et al. 2012). However, Asahara et al. (1997), following isolation of putative endothelial progenitor cells (EPCs) from human peripheral blood using magnetic bead selection, demonstrated that CD34+ cells after seven days of culture started to express markers from endothelial lineage such as CD31, VEGFR-2 and E-Selectin. Those putative EPCs act either directly on the site of injury via migration, proliferation and differentiation or indirectly in a paracrine manner, which in turn results in activation of several pro-angiogenic factors leading to proliferation and migration of pre-existing endothelial cells (Asahara et al. 2011). This critical function of circulating EPCs to promote neovascularisation has brought attention to their role as a surrogate marker of CVD and as a prognostic indicator of cardiovascular events and mortality (Shintani et al. 2001; Vasa et al. 2001; Werner et al. 2005; Lu et al. 2016).
To counterbalance the reduced levels of EPCs and their impaired function in clinical populations, several pharmacological treatments, such as statins and angiotensin converting enzyme inhibitors have been implemented successfully (Sen et al. 2011; Lee and Poh 2014). Moreover, recent studies have reported that lifestyle modifications such as exercise and diet can also increase the number of EPCs and subsequently improve vascular function (Fernandez et al. 2012; De Biase et al. 2013; Maiorino et al. 2017). Accordingly, the latest guidelines for the management of chronic heart failure (CHF) and arterial hypertension recommend regular aerobic exercise as class I and level A of evidence for improvement in symptoms, functional capacity, reduced risk of hospitalisation (McDonagh et al. 2021) and for the reduction of cardiovascular risk and mortality (Williams et al. 2018). Apart from aerobic exercise, the inclusion of resistance exercise 2–3 days per week is also recommended for the management of arterial hypertension (Williams et al. 2018). Combining aerobic and resistance exercise is particularly recommended for the prevention and improved management of type 2 diabetes mellitus (T2DM) (Cosentino et al. 2020). High intensity interval training (HIIT) is another mode of exercise which results in improvements in cardiorespiratory fitness and cardiometabolic health in several populations including overweight and obese individuals (Batacan et al. 2017). Notably, a meta-analysis showed that HIIT can be a better exercise modality compared to moderate intensity continuous exercise (MICON) for the improvement of vascular function and the related biomarkers in clinical populations (Ramos et al. 2015).
Previous systematic reviews (Ribeiro et al. 2013; Palmefors et al. 2014) and meta-analyses (Pearson and Smart 2017; Cavalcante et al. 2019) have investigated the impact of long-term exercise on the number of circulating EPCs in CVD patients only. Nevertheless, little attention was placed on the exercise prescription configurations that induce optimum EPC mobilisation. It is also worth noting that the key pro-angiogenic factors that mediate exercise-induced EPC mobilisation have been systematically extracted and discussed alongside EPCs only in systematic reviews conducted until 2012 (Ribeiro et al. 2013; Palmefors et al. 2014). Finally, the acute and chronic effects of different exercise modalities in populations with impaired metabolic health (i.e., diabetes mellitus and metabolic syndrome) have not been systematically reviewed previously.
Therefore, the primary objective of this review is to systematically investigate the acute and chronic effects of different exercise modalities on circulating EPCs in patients with CVD and metabolic disease. A secondary objective is to investigate the available evidence with regards to the interplay between endothelial function, angiogenic factors and EPCs in individuals with CVD and metabolic diseases across the lifespan.
Methods
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement (Moher et al. 2009) and was prospectively registered in the PROSPERO database (CRD42017084552).
Literature search
A systematic literature search was conducted from 1996 until May 2018 using selected databases (MEDLINE, Cochrane Library (CENTRAL), SPORTdiscus, CINAHL, PsycINFO and SCOPUS). An update of the literature published between May 2018 to February 2020 and February 2020 to April 2021 was conducted using the MEDLINE database. Reference lists of existing reviews and eligible articles were used to assist the identification of eligible articles. Keyword searches for “population”, “exercise” and “endothelial progenitor cells” were performed using Boolean operators and wild cards where appropriate [Details of the strategy can be found in Supplementary Table 1 (S1)] No language restrictions were made. Findings reported here relate to the clinical populations of the registered review in the PROSPERO database however the search strategy included terms for both clinical and healthy populations.
Study selection
The present review included randomised (RCTs) and non-randomised controlled trials (non-RCTs), prospective cohort studies, controlled before—after studies and without control before—after studies in human interventions that investigated the acute and chronic effects of different modes of exercise on circulating EPCs.
Study eligibility was determined by the following key inclusion criteria: (1) inclusion of individuals with CVD, metabolic disease, (2) implementation of structured exercise training programmes or acute exercise training bouts and (3) studies that implemented flow cytometry as main method to enumerate circulating EPCs. The criteria for the EPC phenotype was to include at least one marker of immaturity/stemness (i.e., CD34) and at least one marker that represent endothelial lineage (i.e., KDR) (Fadini et al. 2008a).
Studies not eligible for inclusion: animal studies, human studies where the participants were under 18 years old or pregnant women or individuals who were dieting.
Citations along with abstracts were transferred to EndNote version X9 and duplicates were removed. Two reviewers (PF and MS) independently screened and assessed the title and abstract of all eligible studies.
Data extraction and quality assessment
After reviewing the full paper of all eligible studies, the data were extracted using a standardised extraction sheet in Microsoft excel (Office 365 Plus) by three independent reviewers (PF, CT and MS) and included: (1) Study information (Author, year); (2) Study population (clinical condition, age, sex, fitness status); (3) Exercise intervention (Acute; defined as a single bout of physical activity (Sellami et al. 2018)/Chronic; defined as repeated number of bouts of physical activity during short or long-term period of time (Sellami et al. 2018); (4) Exercise protocol (type of exercise, intensity, duration); (5) Primary outcomes (EPC phenotype, unit of measure, blood sampling time); (6) Secondary outcomes [cytokines, growth factors, chemokines, FMD, maximal oxygen uptake (\(V{\text{O}}_{2\max }\))]. After data extraction, a meeting was held by the three reviewers to cross-check the extracted data. Any disagreements were resolved by discussion. Data not provided in the text or tables were extracted from figures using a semi-automated graph digitizer software (WebPlotDigitizer).
Study quality was assessed by PF and TI, using different critical appraisal tools appropriate for each study design. For the RCTs and non-RCTs, the TESTEX (Tool for the assEssment of Study qualiTy and reporting in Exercise) appraisal tool was used, which is a 15-point scale designed specifically for exercise intervention trials (Smart et al. 2015). For prospective cohort studies, controlled before—after studies and before—after studies the respective quality assessment tools from the National Heart, Lung and Blood institute were used (NHLBI 2021).
Evidence synthesis
A narrative synthesis was undertaken, constructing evidence tables of key study characteristics along with an accompanying narrative synthesis across studies, in accordance with the Synthesis without meta-analysis in systematic reviews: reporting guideline (Campbell et al. 2020). The extracted data presented in two broad categories and in table format: trials that investigated the acute effects of exercise on EPCs and trials that investigated the chronic effects of exercise on EPCs. Tables then were thematically divided based on the exercise modality utilised and the clinical condition. Tables regarding the number of blood collection points and fasting/non-fasting status were arranged in alphabetical order. Quality assessment results were arranged by decreasing order based on the quality score.
The graphical abstract and Fig. 2 were created with BioRender.com. Due to the diversity in study designs, methodological analysis on EPCs (e.g. different phenotypes, different units of measure, different processing and time of blood collection) a meta-analysis was not conducted. Instead, extracted result data along with the P values from the papers were reported to assist the narrative synthesis and interpretation.
Results
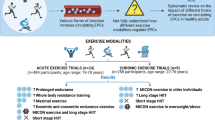
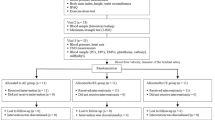
The initial electronic search identified 1388 articles and the manual search from previous reviews another 10, in total 1398 articles. After exclusion of duplicates, 827 articles were reviewed based on title and abstract and after the first sifting 101 potentially eligible articles remained for full-text screening. This resulted in 58 articles that met the inclusion criteria. Following the two updated searches, nine additional eligible articles were identified, increasing the total number of articles to 67. A full list of the 49 articles, accompanied by reason, excluded for this review can be found as Supplementary Table 2 (S2). Figure 1 shows the PRISMA flow diagram of the screening process. Thirsty-six of the articles that met the inclusion criteria were included in this systematic review which focuses on populations with CVD and metabolic abnormalities. The remainder will form the basis for a systematic review that addressing the effects on healthy individuals across the lifespan.
Overview of the study characteristics
The 36 articles yielded 38 trials as one of the publications included three RCT trials (Sandri et al. 2005). The total number of participants was 1731. Thirty-three trials (1588 participants) reported sex distribution with 1247 males (78.5%) and 341 females (21.5%) respectively. Five trials did not report sex distribution (Sandri et al. 2005; Adams et al. 2004; Shaffer et al. 2006).
Acute clinical trial characteristics and intervention details
Fifteen clinical trials examined the acute effects to exercise (Adams et al. 2004; Shaffer et al. 2006; Van Craenenbroeck et al. 2009; Van Craenenbroeck et al. 2010a; Sandri et al. 2011; Van Craenenbroeck et al. 2011; Rummens et al. 2012; Scalone et al. 2013; Kazmierski et al. 2015; Rocha et al. 2015; West et al. 2015; Lutz et al. 2016; Waclawovsky et al. 2016; Gevaert et al. 2019; Kourek et al. 2020b) (Table 1). Thirteen trials included independent groups before and after (Adams et al. 2004; Shaffer et al. 2006; Van Craenenbroeck et al. 2009; Van Craenenbroeck et al. 2010a; Van Craenenbroeck et al. 2011; Rummens et al. 2012; Scalone et al. 2013; Kazmierski et al. 2015; Rocha et al. 2015; West et al. 2015; Lutz et al. 2016; Gevaert et al. 2019; Kourek et al. 2020b), one was a single arm trial (Sandri et al. 2011) and another one a randomised cross over trial (Waclawovsky et al. 2016). Across the 15 included trials (649 participants), 13 reported percentages of male and female distribution (Van Craenenbroeck et al. 2009; Van Craenenbroeck et al. 2010a; Sandri et al. 2011; Van Craenenbroeck et al. 2011; Rummens et al. 2012; Scalone et al. 2013; Kazmierski et al. 2015; Rocha et al. 2015; West et al. 2015; Lutz et al. 2016; Waclawovsky et al. 2016; Gevaert et al. 2019; Kourek et al. 2020b). Across these 13 trials (573 participants), 435 were males (75.9%) and 138 were females (24.1%). A healthy exercise control group was included in 12 out of the 15 acute clinical trials (Adams et al. 2004; Shaffer et al. 2006; Van Craenenbroeck et al. 2010a; Van Craenenbroeck et al. 2011; Rummens et al. 2012; Scalone et al. 2013; Kazmierski et al. 2015; Rocha et al. 2015; West et al. 2015; Lutz et al. 2016; Waclawovsky et al. 2016; Gevaert et al. 2019). The total healthy control were 199 participants (33.7%), whereas the total clinical population was 450 participants (69.3%). The age of the clinical population ranged from 27 to 74 and of the healthy controls from 27 to 73 years old. The clinical conditions for the acute trials included: four with CHF with reduced ejection fraction (HFrEF) and mid-ranged ejection fraction (HFmrEF) (Van Craenenbroeck et al. 2009, 2010a, 2011; Kourek et al. 2020b), one CHF with preserved ejection fraction (HFpEF) (Gevaert et al. 2019), four with coronary artery disease (CAD) and microvascular angina (Adams et al. 2004; Rummens et al. 2012; Scalone et al. 2013; Kazmierski et al. 2015), two with peripheral arterial disease (PAD) (Shaffer et al. 2006; Sandri et al. 2011), two with T1DM (West et al. 2015; Waclawovsky et al. 2016), one with T2DM and impaired glucose tolerance (Lutz et al. 2016) and one with early metabolic syndrome (MetS) (Rocha et al. 2015).
The most common exercise mode for studying the acute effects was a cardiopulmonary exercise test (CPET) on a cycle ergometer (Adams et al. 2004; Van Craenenbroeck et al. 2009, 2010a, 2011; Rummens et al. 2012; Gevaert et al. 2019; Kourek et al. 2020b). Three trials utilised a standard Bruce test and a Gardner–Skinner stress test on a treadmill (Shaffer et al. 2006; Scalone et al. 2013; Kazmierski et al. 2015), and one trial utilised a symptom-limited maximal test on a treadmill (Sandri et al. 2011). With regards to MICON exercise on a cycle ergometer, intensity ranged from 60 to 70% \(V{\text{O}}_{2\max }\) and duration between 30 and 45 min in two trials (West et al. 2015; Lutz et al. 2016), and one trial prescribed 40 min of cycling at 80% of the ventilatory threshold (Rocha et al. 2015). Finally, one trial compared a MICON protocol with a lower limb resistance exercise protocol matched in total duration as the MICON (Waclawovsky et al. 2016).
In total, eleven different phenotypes were used to identify circulating EPCs in the acute trials. The most common antibody combination was CD34+/KDR+ (Adams et al. 2004; Shaffer et al. 2006; Van Craenenbroeck et al. 2009; Sandri et al. 2011; Rummens et al. 2012; Rocha et al. 2015; Lutz et al. 2016). The remaining trials used: CD34+/CD133+/KDR+ (5 trials) (Shaffer et al. 2006; Rummens et al. 2012; Kazmierski et al. 2015; Rocha et al. 2015; Kourek et al. 2020b), CD34+/KDR+/CD45dim (3 trials) (Gevaert et al. 2019; Waclawovsky et al. 2016; West et al. 2015), CD34+/KDR+/CD3− (2 trials) (Van Craenenbroeck et al. 2010a, 2011), CD133+/KDR+ (2 trials) (Shaffer et al. 2006; Sandri et al. 2011), CD34+/CD133−KDR+ (1 trial) (Rummens et al. 2012) and CD34+/KDR+/CD45− (Scalone et al. 2013) (1 trial). Finally, two trials used two versions of four antibody combinations including CD133+/CD34+/KDR+/CD31− or CD34+/KDR+/CD146+/CD31− (Shaffer et al. 2006) and CD34+/CD45−/CD133+/KDR+ or CD34+/CD45−/CD133−/KDR+ (Kourek et al. 2020b) respectively. Large variability was observed in terms of the units used to express EPCs. The most common units were the absolute number of cells (cells/mL or cells/μL) (Adams et al. 2004; Sandri et al. 2011; Rummens et al. 2012; Kazmierski et al. 2015) and cells per 106 events (Van Craenenbroeck et al. 2010a, 2011; Lutz et al. 2016). Several other units of EPCs identified, such as percentage lymphocytes (Van Craenenbroeck et al. 2009), cells per 106 mononuclear cells (Gevaert et al. 2019), cells per 105 mononuclear cells (Scalone et al. 2013), percentage of live events (Shaffer et al. 2006), cells per 100 leucocytes (West et al. 2015), percentage cells in the CD34+ gate (Rocha et al. 2015) and cells per 106 enucleated cells (Kourek et al. 2020b). One trial presented EPCs in logarithmic scale (Waclawovsky et al. 2016). Lastly, as a secondary outcome four trials measured the number and/or function of cultured myeloid angiogenic cells (MACs) defined as double positive for acetylated Low-Density Lipoprotein labelled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate and lectin (Di-acLDL + /lectin + cells) (Adams et al. 2004; Van Craenenbroeck et al. 2009, 2010a; Sandri et al. 2011) [Supplementary Table 3 (S3)].
Ten out of the 15 trials (Table 2) predominantly included two blood collection time points for circulating EPCs with the post-exercise time point varying between immediately post-exercise and 24 h post-exercise (Shaffer et al. 2006; Van Craenenbroeck et al. 2009; Van Craenenbroeck et al. 2010a; Rummens et al. 2012; Scalone et al. 2013; Rocha et al. 2015; Lutz et al. 2016; Waclawovsky et al. 2016; Gevaert et al. 2019; Kourek et al. 2020b). The most common time point adopted in the majority of trials was 10 min post-exercise. Two trials included three time points (Kazmierski et al. 2015; West et al. 2015). Three trials investigated the kinetics of circulating EPCs after exercise, whereas one included seven time points up to 48 h post-exercise (Adams et al. 2004), and two had 10 time points up to 48 h and 72 h post-exercise respectively (Sandri et al. 2011; Van Craenenbroeck et al. 2011). Four trials reported fasting (Shaffer et al. 2006; Rocha et al. 2015; Lutz et al. 2016; Waclawovsky et al. 2016), and three trials reported non-fasting blood samples (Van Craenenbroeck et al. 2010a, 2011; Rummens et al. 2012). One trial reported pre-exercise non-fasting blood sampling and the ~ 15 h post-exercise fasting blood sample (West et al. 2015). Finally, seven trials did not report any details (Adams et al. 2004; Van Craenenbroeck et al. 2009; Sandri et al. 2011; Scalone et al. 2013; Kazmierski et al. 2015; Gevaert et al. 2019; Kourek et al. 2020b) (Table 2).
Chronic clinical trial characteristics and intervention details
From the 38 clinical trials, 23 examined the chronic effects of exercise on circulating EPCs (Laufs et al. 2004; Sandri et al. 2005, 2016; Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009, 2013; Erbs et al. 2010; Van Craenenbroeck et al. 2010b, 2015; Hansen et al. 2011; Schlager et al. 2011; Gatta et al. 2012; Luk et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b; Gagliardi et al. 2016; Jo et al. 2020; Kourek et al. 2020a) (Table 3). Ten trials were RCTs (Sandri et al. 2005, 2016; Erbs et al. 2010; Schlager et al. 2011; Luk et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Gagliardi et al. 2016), six single arm before—after trials (Laufs et al. 2004; Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009, 2013; Gatta et al. 2012), four randomised trials (Hansen et al. 2011; Van Craenenbroeck et al. 2015; Jo et al. 2020; Kourek et al. 2020a) and three non-RCTs (Steiner et al. 2005; Van Craenenbroeck et al. 2010b; Dopheide et al. 2016b). Across the 23 included trials (1082 participants), 20 trials reported percentage of female and male distribution (Laufs et al. 2004; Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009, 2013; Erbs et al. 2010; Van Craenenbroeck et al. 2010b, 2015; Hansen et al. 2011; Schlager et al. 2011; Gatta et al. 2012; Luk et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b; Gagliardi et al. 2016; Sandri et al. 2016; Jo et al. 2020; Kourek et al. 2020a). Across these 20 trials (1015 participants), 812 (80.0%) were males and 203 (20%) were females. In chronic clinical interventions, age ranged from 44 to 88 years old. In chronic interventions, the clinical conditions included: HFrEF (8 trials) (Sarto et al. 2007; Erbs et al. 2010; Van Craenenbroeck et al. 2010b; Gatta et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Sandri et al. 2016; Kourek et al. 2020a), CAD (8 trials) (Laufs et al. 2004; Sandri et al. 2005; Steiner et al. 2005; Paul et al. 2007; Hansen et al. 2011; Luk et al. 2012; Van Craenenbroeck et al. 2015; Gagliardi et al. 2016), PAD (4 trials) (Sandri et al. 2005; Schlager et al. 2011; Dopheide et al. 2016b), cardiac patients (coronary artery bypass graft and valve replacement) (1 trial) (Cesari et al. 2009), acute coronary syndrome (ACS) (1 trial) (Cesari et al. 2013), and hypertensive MetS (1 trial) (Jo et al. 2020).
Of the 23 chronic clinical trials, 13 trials utilised MICON exercise (cycling or walking) (Sandri et al. 2005; Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011; Gatta et al. 2012; Cesari et al. 2013; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b), seven investigated a MICON protocol combined with either resistance exercise (Laufs et al. 2004; Van Craenenbroeck et al. 2010b; Hansen et al. 2011; Luk et al. 2012) or calisthenics (Cesari et al. 2009; Gagliardi et al. 2016; Sandri et al. 2016), two compared MICON vs HIIT (Van Craenenbroeck et al. 2015; Jo et al. 2019) and one compared HIIT vs HIIT combined with resistance exercise (Kourek et al. 2020a). The intervention duration ranged from two to 32 weeks, with weekly training frequency two to seven times per week and session duration from 10 to 60 min.
Eight different EPC phenotypes were identified for the quantification of circulating EPCs by flow cytometry. The most common phenotype used was CD34+/KDR+ (13 trials) (Sandri et al. 2005, 2016; Cesari et al. 2009, 2013; Erbs et al. 2010; Hansen et al. 2011; Gatta et al. 2012; Luk et al. 2012; Gagliardi et al. 2016; Jo et al. 2020), followed by CD34+/KDR+/CD133+ (6 trials) (Steiner et al. 2005; Cesari et al. 2009, 2013; Schlager et al. 2011; Hansen et al. 2011; Kourek et al. 2020a), CD34+/KDR+/CD45dim (4 trials) (Eleuteri et al. 2013; Mezzani et al. 2013; Van Craenenbroeck et al. 2015; Dopheide et al. 2016b), CD133+/KDR+ (4 trials) (Paul et al. 2007; Cesari et al. 2009, 2013; Sandri et al. 2016), CD34+/KDR+/CD31+ (1 trial) (Sarto et al. 2007), and CD34+/KDR+/CD3− (1 trial) (Van Craenenbroeck et al. 2010b). One trial used two versions of four antibody combinations including CD34+/CD45−/CD133+/KDR+ or CD34+/CD45−/CD133−/KDR+ (Kourek et al. 2020a). The units of measure for EPCs were also variable. Nine trials reported absolute values (cells/mL or cells/μL) (Sandri et al. 2005, 2016; Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009; Erbs et al. 2010; Hansen et al. 2011), three trials percentage of mononuclear cells (Schlager et al. 2011; Eleuteri et al. 2013; Mezzani et al. 2013), three trials percentage of positive cells (Steiner et al. 2005; Gatta et al. 2012; Dopheide et al. 2016b), two trials cells per 106 events (Van Craenenbroeck et al. 2010b; Cesari et al. 2013), two trials cells per 105 events (Laufs et al. 2004; Gagliardi et al. 2016), one trial cells per 106 mononuclear cells (Van Craenenbroeck et al. 2015) and one trial cells per 106 enucleated cells (Kourek et al. 2020a). Lastly, of the 23 chronic trials, nine trials examined the number and/or function of cultured MACs defined as Di-acLDL + /lectin + cells (Laufs et al. 2004; Sandri et al. 2005, 2016; Sarto et al. 2007; Erbs et al. 2010; Van Craenenbroeck et al. 2010b; Schlager et al. 2011) (Supplementary Table S3).
Seventy-eight percent of the trials (n = 18) included two blood sampling time points (pre- and post-intervention), three trials five time points (Sandri et al. 2005), and two trials three time points (Schlager et al. 2011; Gagliardi et al. 2016). The post-training blood samples were drawn 48 h after the last training session in five trials (Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009, 2013), 24 h in one trial (Gatta et al. 2012), 24—48 h in one trial (Gagliardi et al. 2016), 72 h in three trials (Sandri et al. 2005), and between 3 – 7 days in one trial (Van Craenenbroeck et al. 2015). Eleven trials did not provide any information regarding timing of blood collection (Table 4) (Laufs et al. 2004; Erbs et al. 2010; Van Craenenbroeck et al. 2010b; Hansen et al. 2011; Luk et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b; Sandri et al. 2016; Jo et al. 2020; Kourek et al. 2020a). Blood samples in 13 trials were drawn in a fasted state (Steiner et al. 2005; Cesari et al. 2009, 2013; Erbs et al. 2010; Van Craenenbroeck et al. 2010b, 2015; Hansen et al. 2011; Schlager et al. 2011; Gatta et al. 2012; Luk et al. 2012; Eleuteri et al. 2013; Dopheide et al. 2016b; Jo et al. 2020), while ten trials reported no information regarding fasting status (Table 4) (Laufs et al. 2004; Sandri et al. 2005, 2016; Paul et al. 2007; Sarto et al. 2007; Mezzani et al. 2013; Gagliardi et al. 2016; Kourek et al. 2020a).
Quality assessment
Eighteen trials were assessed with the TESTEX scale (Sandri et al. 2005, 2016; Steiner et al. 2005; Erbs et al. 2010; Van Craenenbroeck et al. 2010b, 2015; Hansen et al. 2011; Schlager et al. 2011; Luk et al. 2012; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b; Gagliardi et al. 2016; Waclawovsky et al. 2016; Jo et al. 2020; Kourek et al. 2020a). The mean TESTEX score was 9.5 out of 15 (standard deviation 2.8), with a range of 5 – 13 (Table 5). Most of the trials did not report allocation concealment and activity monitoring in control group. Intention to treat analysis was not considered in any of the included trials.
The acute trials that included independent groups were assessed with the observational cohort and cross-sectional studies appraisal tool (Table 6). Two acute trials were classified as “good” (Kazmierski et al. 2015; Gevaert et al. 2019), ten as “fair” (Adams et al. 2004; Van Craenenbroeck et al. 2009; Van Craenenbroeck et al. 2010a; Van Craenenbroeck et al. 2011; Rummens et al. 2012; Scalone et al. 2013; Rocha et al. 2015; West et al. 2015; Lutz et al. 2016; Kourek et al. 2020b) and one as “poor” (Shaffer et al. 2006). The majority of the trials received a “No” in sample size justification, different levels of exposure and repeated exposure of assessment questions respectively.
Seven single arm trials assessed with the Before-After (Pre-Post) studies with no control group appraisal tool (Table 7). Of those, six were classified as “good” (Paul et al. 2007; Sarto et al. 2007; Cesari et al. 2009, 2013; Sandri et al. 2011; Gatta et al. 2012) and one as “fair” (Laufs et al. 2004). The blinding of assessors could not be determined in the majority of the trials, with in only one trial being clearly described (Sarto et al. 2007).
Acute effects on circulating EPCs and angiogenic factors
Table 2 shows the acute effects in circulating EPCs and angiogenic factors. When examining the acute effects using CPET on a cycle ergometer two trials found no changes in circulating EPCs in both the CHF patients and healthy control groups (Van Craenenbroeck et al. 2009, 2010a; Gevaert et al. 2019). However, two of those trials found that the migratory capacity of Di-acLDL + /lectin + MACs towards VEGF and SDF-1α was significantly improved after exercise (Van Craenenbroeck et al. 2009, 2010a). In addition, the improvement in MACs migratory capacity was higher (52%) in more severe CHF patients compared to those with mild-CHF (31%), whereas a small reduction was observed in the aged match healthy control group (Van Craenenbroeck et al. 2010a). Another trial found CHF patients (HFrEF and HFmrEF) irrespective of their disease severity based on median \(V{\text{O}}_{{2{\text{peak}}}}\) (≥ 18 mL kg−1 min−1 or < 18 mL kg−1 min−1), median minute ventilation—carbon dioxide production relationship (VE/VCO2 slope) (≥ 32.5 or < 32.5) or left ventricular ejection fraction (≥ 40% or < 40%) were able to mobilise circulating CD34+/CD45−CD133+/KDR+ and CD34+/CD45−/CD133−/KDR+ EPCs immediately post-exercise (Kourek et al. 2020b). In the same trial, CHF patients with increased disease severity, based on the aforementioned criteria, were able to mobilise CD34+/CD133+/KDR+ EPCs as well as compared to the group with reduced disease severity (Kourek et al. 2020b). One trial reported an increase in EPCs in both their old and young healthy groups but not in the HFrEF group (Van Craenenbroeck et al. 2011). In another trial, there was an increase in CD34+/KDR+ and CD34+/CD133−/KDR+ EPCs in both the CAD and the healthy groups (Rummens et al. 2012). However, the increase in CD34+/CD133−/KDR+ EPCs was more prominent in the healthy group (Rummens et al. 2012). Finally, one trial (Adams et al. 2004) reported that a symptom-limited test using a cycle ergometer resulted in a significant elevation of CD34+/KDR+ EPCs by 164% at 24 h and by 76% at 48 h in an ischaemic CAD group. In the same trial the changes on circulating EPCs were accompanied by a 2.9 ± 0.4-fold and 3.3 ± 0.5-fold increase in Di-acLDL + /lectin + MACs at 24 h and 48 h respectively (Adams et al. 2004). Two trials assessed vascular endothelial growth factor (VEGF) with one reporting an increase in an ischaemic CAD group (Adams et al. 2004) and the other trial reported no change either in HFrEF or healthy groups (Van Craenenbroeck et al. 2010a). Circulating levels of stromal-cell derived factor one alpha (SDF-1α) was measured in two trials (Van Craenenbroeck et al. 2010a, 2011), with one showing changes in the mild HFrEF and healthy groups but not in the severe HFrEF group (Van Craenenbroeck et al. 2010a). The second one did not report any changes (Van Craenenbroeck et al. 2011). Finally, one trial measured granulocyte macrophage colony stimulating factor (GM-CSF) and basic fibroblast growth factor, but no changes were reported (Adams et al. 2004).
In the trials that incorporated various stress test protocols on a treadmill, one trial found an increase in both CD34+/KDR+ and CD133+/KDR+ EPCs by 212% and 278% respectively; this was in turn accompanied by a 230.6% increase in Di-acLDL + /lectin + MACs and a 361% increase in serum VEGF in stable PAD patients, but no changes in GM-CSF and basic fibroblast growth factor (Sandri et al. 2011). In a second trial (Shaffer et al. 2006) no effects were observed in various EPC phenotypes in a group of PAD patients. Nevertheless, significant reductions of CD133+/CD34+/KDR+/CD31− EPCs by 63% were evident in the age-matched healthy group.
From the trials that incorporated a MICON protocol, one trial (Lutz et al. 2016) reported a 23% increase on CD34+/KDR+ EPCs in a normal glucose tolerance group, while no changes were observed in the impaired glucose tolerance and T2DM groups. In another trial (West et al. 2015) increase in CD34+/KDR+/CD45dim EPCs after ~ 15 h post-exercise was significant in the healthy group but not in the T1DM group. In a third trial (Rocha et al. 2015) the early MetS group had a significant reduction in CD34+/KDR+ and CD133+/CD34+/KDR+ EPC levels after a bout of MICON compared with their age-matched healthy control group. The latter group increased matrix metalloproteinase (MMP)-2 levels post-exercise while both groups had an increase in granulocyte colony stimulating factor. Moreover, MMP-9 levels increased significantly post-exercise in the early MetS group. VEGF and GM-CSF remained unchanged in both groups.
Lastly, the only trial (Waclawovsky et al. 2016) that compared a MICON cycling bout with a lower limb resistance protocol reported no effect in T1DM patients. In contrast, the healthy control group had a significant increase of EPCs 10 min after the resistance protocol and a significant reduction after the moderate intensity cycling bout.
Chronic effects on EPCs, fitness status, angiogenic factors, and endothelial function
Table 3 reports the chronic effects of exercise on EPCs, fitness status, angiogenic factors, and endothelial function in clinical populations. Of the 13 trials that included MICON exercise, 10 found significant increases in EPC levels (Sandri et al. 2005; Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011; Gatta et al. 2012; Cesari et al. 2013; Eleuteri et al. 2013; Mezzani et al. 2013), two reported no change (Sandri et al. 2005), and one reported more pronounced and significant reduction in a supervised exercise group compared with the non-supervised exercised group (Dopheide et al. 2016b). In four studies, six trials included a cultivation of MACs to assess their number and/or their function (Sandri et al. 2005; Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011). Di-acLDL + /lectin + MACs were analysed in 5 of these trials, with three reporting significant increases in their numbers (Sandri et al. 2005; Sarto et al. 2007; Schlager et al. 2011) and two reporting no change (Sandri et al. 2005). Regarding MACs migratory capacity, one trial reported increase by 107.1% (Erbs et al. 2010) and another, increase at 3 and 6 months respectively (Schlager et al. 2011). Finally, three trials reported improvements of MACs ability to participate in network formation with no change to the controls (Sandri et al. 2005).
All 13 trials reported a significant increase in fitness status after the intervention (Sandri et al. 2005; Steiner et al. 2005; Paul et al. 2007; Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011; Gatta et al. 2012; Cesari et al. 2013; Eleuteri et al. 2013; Mezzani et al. 2013; Dopheide et al. 2016b) with two trials reporting a significant positive correlation between the increases in EPCs and \(V{\text{O}}_{{2{\text{peak}}}}\) (Cesari et al. 2013; Mezzani et al. 2013). Only four trials assessed endothelial function by FMD (Steiner et al. 2005; Paul et al. 2007; Erbs et al. 2010; Eleuteri et al. 2013) with two trials showing improvements (Erbs et al. 2010; Eleuteri et al. 2013) and the other two no changes in FMD (Steiner et al. 2005; Paul et al. 2007). In one of those trials, there was a trend for in improvements in FMD (P = 0.07), which was associated with a strong and significant positive correlation between ΔFMD with ΔEPCs: (r = 0.81, P < 0.01) (Steiner et al. 2005). With respect to circulating angiogenic factors, nine out of the 13 trials measured serum or plasma VEGF (Sandri et al. 2005; Steiner et al. 2005; Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011; Eleuteri et al. 2013; Dopheide et al. 2016b). Five of them reporting a significant increase (Sandri et al. 2005; Steiner et al. 2005; Sarto et al. 2007; Erbs et al. 2010; Dopheide et al. 2016b) where one reported a positive relationship between VEGF and EPCs (r = 0.66, P < 0.05) (Sandri et al. 2005) and one reported an inverse relationship between individual changes in VEGF with changes in EPCs (r = – 0.477, P < 0.001). Four trials reported no significant changes in any of the primary outcome measures (Sandri et al. 2005; Schlager et al. 2011; Eleuteri et al. 2013). Three trials evaluated the levels of SDF-1α (Sarto et al. 2007; Erbs et al. 2010; Schlager et al. 2011; Eleuteri et al. 2013), with two of them reporting an increase after the intervention (Sarto et al. 2007; Erbs et al. 2010). Three trials measured GM-CSF levels, which remained unchanged at post-intervention (Sandri et al. 2005). One trial measured angiopoietin 1 (Ang-1) and angiopoietin 2 (Ang-2) with significant increases only in the latter. Furthermore, no significant relationships between EPCs, Ang 1, and Ang 2 were observed (Eleuteri et al. 2013). Only one trial assessed MMPs and found no changes in MMP-2 and MMP-9. Interestingly, there was an increase in the ratio of MMPs over the tissue inhibitor of metalloproteinase 1 (MMP-2/TIMP-1 and MMP-9/TIMP-1) respectively (Gatta et al. 2012).
From the four trials that combined resistance and MICON exercise, two observed significant increases in EPC levels (Laufs et al. 2004; Van Craenenbroeck et al. 2010b), which were paralleled with improvements in MACs migratory capacity by 77% (Van Craenenbroeck et al. 2010b), and a reduced rate of apoptosis (Laufs et al. 2004). Furthermore, exercise capacity was improved in three trials (Laufs et al. 2004; Hansen et al. 2011; Luk et al. 2012), whereas in the fourth one the \(V{\text{O}}_{{2{\text{peak}}}}\) was not improved (Van Craenenbroeck et al. 2010b). Two trials investigated endothelial function and found improvements in FMD following completion of the combined protocols (Van Craenenbroeck et al. 2010b; Luk et al. 2012).
Of the three trials (Cesari et al. 2009; Gagliardi et al. 2016; Sandri et al. 2016) that implemented MICON exercise and calisthenics, only one found significant increases in EPC levels, which was also accompanied by increases in in MACs migratory capacity, in FMD and SDF-1α (Sandri et al. 2016). Regarding circulating VEGF levels, one trial found an increase (Sandri et al. 2016) whereas another one did not report any changes (Gagliardi et al. 2016) and one reported significant reduction (Cesari et al. 2009).
In the two trials that compared MICON vs a HIIT protocol (Van Craenenbroeck et al. 2015; Jo et al. 2020), one found no changes to circulating EPCs regardless of the protocol followed, (Van Craenenbroeck et al. 2015), While the second one found an 88% increase in EPCs in the HIIT group. This increase was also accompanied by improvements in FMD and an increase in nitric oxide (NOx) metabolites (Jo et al. 2020). In one of the trials (Van Craenenbroeck et al. 2015), \(V{\text{O}}_{{2{\text{peak}}}}\) was increased significantly following completion of both training protocols. Regarding endothelial function, vascular FMD was significantly improved in both the HIIT and MICON groups (Van Craenenbroeck et al. 2015; Jo et al. 2020), with greater improvements in the HIIT group (Jo et al. 2020). One trial, which compared HIIT vs HIIT combined with resistance training in CHF patients, found significant increases in all three EPC subpopulations without any significant differences between the exercise protocols. In both protocols there was a significant improvement in \(V{\text{O}}_{{2{\text{peak}}}}\), an increase in circulating VEGF and a reduction in C-reactive protein (CRP) (Kourek et al. 2020a).
Discussion
The primary aim of this review was to investigate the acute and chronic effects of different exercise modalities on circulating EPCs in patients with CVD and metabolic abnormalities. A secondary aim was to identify putative mechanisms of exercise-induced EPC mobilisation and possible links between EPCs and endothelial function as assessed by FMD and aerobic capacity.
In CHF patients, despite the majority of the research indicating that acute EPC mobilisation in HFrEF and HFpEF patients is blunted following completion of a CPET on a cycle ergometer, despite an improvement in MACs migratory capacity, concrete conclusions cannot be made. On the other hand, EPC mobilisation and enhancement of MACs migratory capacity was evident in ischaemic and revascularized CAD completing either a CPET using cycle ergometry or a maximal treadmill exercise test. In PAD patients following a symptom-limited maximal exercise test were equivocal. In patients with altered metabolic health, such as DM, have an impaired ability to mobilise EPCs following completion of an acute exercise bout when compared with healthy age-matched controls.
In the chronic trials, there was strong support for the utility of different exercise training modes for increasing circulating EPCs, irrespective of the phenotype used for EPC identification, in HFrEF and ACS patients. The increase in circulating EPCs showed to be accompanied by increase in MACs ability to migrate. Results were equivocal in CAD patients whereas PAD patients with greater disease severity benefited the most in terms of EPC mobilisation following completion of a chronic exercise training intervention. Long-term training studies provide support for the superiority of HIIT over MICON regarding EPC mobilisation in hypertensive patients with metabolic syndrome.
Angiogenic factors that were more frequently assessed included VEGF, SDF-1α and GM-CSF with evidence suggesting that exercise may have some positive effects on VEGF and SDF-1α. However, there was limited evidence to suggest that observed significant improvements in FMD and aerobic capacity were associated with EPC mobilisation. Collectively our findings suggest that, regardless of exercise mode, chronic training interventions can be an effective means of improving cardiometabolic health. Finally, there is a need for additional research to confirm the long-term impact of exercise on EPC mobilisation in CAD patients, while HIIT training in the long term may be of greater benefit in hypertensive metabolic syndrome patients.
Acute effects of exercise on EPCs
In HFrEF patients, EPC mobilisation is mostly impaired following completion of CPET protocols involving cycle ergometry (Van Craenenbroeck et al. 2009, 2010a, 2011). In addition, circulating EPCs did not change in the HFrEF group 48 h post-exercise when compared with either a healthy older or healthy younger group (Van Craenenbroeck et al. 2011). However, only one trial found a significant increase in EPC populations after CPET in CHF patients (Kourek et al. 2020b). However, the latter trial included HFmrEF patients in addition to HFrEF patients, who tend to have a higher mean left ventricular ejection fraction. We postulate that the observation that most trials did not find any increase could be partly due to underlying ischaemic factors that can lead to the exhaustion of progenitor cells from the bone marrow (Kissel et al. 2007). It is noteworthy that HFrEF patients’ baseline EPC levels follow a biphasic pattern: at the early stages of the syndrome, there is an increased number (possibly to counterbalance endothelial injury), while in the more advanced stages of the syndrome there seems to be a depletion of the EPC pool (Valgimigli et al. 2004). Consequently, for patients at the early stages of the syndrome, with an already increased baseline EPC number, exercise may not increase EPCs any further due to the already exhausted bone marrow EPC pool (Van Craenenbroeck et al. 2011). The short duration of CPET protocols (i.e., 8–12 min on average) may not be adequate to optimally stimulate the mobilisation of EPCs since in a recent trial in CHF patients both a HIIT of longer duration (4sets of 4 min at 80%\(V{\text{O}}_{{2{\text{peak}}}}\) with 3 min at 50%\(V{\text{O}}_{{2{\text{peak}}}}\)) and a MICON (50%\(V{\text{O}}_{{2{\text{peak}}}}\)) protocols matched for total work acutely increased circulating EPC levels immediately after and 40 min post-exercise (Mitsiou et al. 2020). In addition, participants in the CHF group with increased disease severity were able to mobilise CD34+/CD133+/KDR+ EPCs compared to participants in the CHF group with reduced disease severity. In healthy individuals, it has been shown that longer exercise times (30 min at either high or moderate intensity) can elicit increases in CD34+/KDR+ EPCs while short duration exercise (10 min) at moderate intensity failed to (Laufs et al. 2005). In addition, the disparity in the results between the trials included CHF patients could also be accounted to the methodological differences for the EPC quantification such as the selection of EPC phenotype. For example, the trials that did not find any alterations on circulating EPCs used either CD34+/KDR+/CD3− or CD34+/KDR+ antibody combination (Van Craenenbroeck et al. 2010a, 2011, 2009) whereas the trial which find increases in EPCs used CD34+/CD45−/CD133+/KDR+, CD34+/CD133+/KDR+ and CD34+/CD45−/CD133−/KDR+ antibody combinations (Kourek et al. 2020b). Finally, the beneficial effects on angiogenesis after a maximal CPET could possibly derive indirectly by the improvement in MACs migratory capacity (Van Craenenbroeck et al. 2009, 2010a). MACs are monocyte macrophage derived cells that do not differentiate to endothelial cells but play an important role in angiogenesis by releasing angiogenic factors such as IL-8, MMP-9 and VEGF (Medina et al. 2011; Chambers et al. 2013). It is worth noting that the most severe CHF patients had larger increase in MACs migratory capacity (52%) compared to their mild counterparts (31%) (Van Craenenbroeck et al. 2010a). Those results are confirmed by the same authors who found a positive relationship between the baseline NT-pro BNP levels and the exercise-induced MACs migratory capacity (r = 0.258, P = 0.001).
It appears that HFpEF patients have an impaired ability to mobilise EPCs in response to maximal exercise (Gevaert et al. 2019); however only one blood sample was drawn 10 min post-exercise (CPET) and therefore, future research is required to assess the time course on EPC mobilisation in HFpEF before concrete conclusions can be made.
In CAD patients, post-exercise the number of EPCs increases regardless of the mode of exercise employed, as this was evidenced following completion of a maximal exercise test on a treadmill (Kazmierski et al. 2015) or a cycle ergometer (Adams et al. 2004; Rummens et al. 2012). Adams et al. (Adams et al. 2004) provided evidence about the role of exercise-induced ischaemia on EPC mobilisation. They showed that only the ischaemic CAD group had an increase in EPC levels (including cultured MACs) with no changes observed in the non-ischaemic CAD and healthy aged-matched groups, respectively. However, two other trials demonstrated that EPCs can also be mobilised in revascularized stable CAD patients after maximal exercise test (Rummens et al. 2012; Kazmierski et al. 2015). This observation suggests that acute maximal-intensity exercise mobilises circulating EPCs in both ischaemic CAD and in CAD patients with a restored myocardial perfusion. Despite these positive findings, exercise-induced EPC mobilisation in CAD patients is either reduced (Rummens et al. 2012) or delayed (Kazmierski et al. 2015) when compared to age-matched healthy controls. This further highlights the role of preventative strategies as well as the need for further research into identifying the optimal exercise modes and exercise doses. These will need to be determined with reference to disease severity to optimise EPC mobilisation. Indeed, increased CAD severity (based on the number of stenotic lesions) negatively affects the exercise-induced EPC mobilisation (Kazmierski et al. 2015). This further highlights the need for personalised exercise prescription based on the severity of the disease.
The trials that investigated the acute effects after a maximal exercise test on a treadmill in PAD patients produced equivocal findings. Sandri et al. (2011) noted an increase in circulating EPCs 24 h after exercise. This was accompanied by an increase in plasma VEGF as well. In contrast, Shaffer et al. (2006) did not observe any change in any of the five EPC phenotypes studied. Possible reasons were that in the latter trial only three out of the 15 patients were able to complete 10 min of exercise and therefore, for most of the cohort, there was insufficient time for the exercise stimulus to provoke changes in EPCs. Considering that Sandri et al. (2011) observed that both CD133+/KDR+ and CD34+/KDR+ EPCs and Di-acLDL + /lectin + MACs peaked at 24 h post-exercise, Shaffer et al. (2006) possibly failed to capture any changes of EPCs since the time point of blood post-exercise collection was at 10 min.
Evidence from different exercise regimes exists in patients with metabolic disease. One trial examined the acute effects of circulating EPCs in early MetS in comparison to a healthy aged-matched control (Rocha et al. 2015). After 40 min of cycling at 80% of ventilatory threshold there was no change in circulating EPCs compared to baseline in both groups. Post-exercise EPC levels were significantly lower in the MetS group compared to the healthy controls. VEGF and GM-CSF also remained unchanged. A possible explanation for the findings is the sustained increase of MMP-9 levels both at rest (Goncalves et al. 2009) and post-exercise (Rocha et al. 2015) that characterises MetS patients. MMP-9’s crucial role in homing of EPCs (Huang et al. 2009) possibly led to a liberation of EPCs in the circulation followed by their migration to the sites of endothelial injury which led to reduced numbers in circulation.
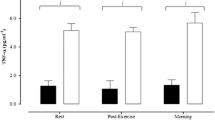
Acute exercise-induced EPC mobilisation is also altered in T1DM patients. Neither a MICON protocol (West et al. 2015) nor a lower limb resistance exercise bout (Waclawovsky et al. 2016) led to a change in circulating EPCs compared to healthy aged-matched controls. T1DM patients are characterised by increased inflammation and oxidative stress (Devaraj et al. 2006). West et al. (2015) found an inverse relationship between TNF-a and the ΔEPC 15 h post-exercise (r = – 0.766, P = 0.005). Previous trials showed that TNF-a has inhibitory effects on EPCs by decreasing ex-vivo cultured EPCs through activation of the p38 MAP kinase pathway (Seeger et al. 2005), increasing EPC apoptosis, reducing inducible nitric oxide synthase and endothelial nitric oxide synthase (eNOS) in cultured EPCs (Chen et al. 2011). Furthermore, it was found that glycated haemoglobin was negatively correlated with the EPC changes 15 h post-exercise.
(r = – 0.65, P = 0.021) (West et al. 2015). Previously, in a large cohort trial of children with T1DM (n = 190), reduced glycated haemoglobin levels were the strongest independent predictor of increase in circulating EPCs after a year of follow up (Hortenhuber et al. 2013). Therefore, there is an argument for the use of strategies to increase exercise levels in an attempt to lower inflammation and TNF-a levels and achieve better glycaemic control; these might reverse the blunted EPC response.
Similarly, the EPC responses of older individuals with T2DM or impaired glucose tolerance were blunted compared to the normal glucose tolerance group (Lutz et al. 2016). The authors proposed that the blunted responses of CD34+/VEGFR2+ EPCs can be accounted for by the impaired ability to increase VEGFR2+ cells since the three groups did not differ in CD34+ numbers.
To conclude, further research is required to identify the optimum mode, duration and intensity of exercise that can acutely mobilise circulating EPCs in patients with CHF and PAD considering that the included trials in this systematic review incorporated a maximal exercise test. Furthermore, despite the evidence that maximal exercise mobilises EPCs in patients with CAD regardless of the mode of exercise (i.e., cycling or running), no data yet exist regarding any other exercise intensities or other forms of exercise (e.g. resistance exercise or HIIT). Limited evidence exists regarding the acute effects of exercise in patients with metabolic disease and this area needs further investigation. Finally, more research is required to compare different exercise regimes with several collection time points to capture the time course of EPC level in circulation.
Chronic effects of exercise on EPCs
The present systematic review provides concrete evidence that MICON type interventions have beneficial effects on circulating EPC levels. In particular, patients with HFrEF compared to PAD and CAD patients benefit more from this type of exercise irrespective of EPC phenotype (CD34+/KDR+, CD34+/KDR+/CD45dim, CD34+/KDR+/CD31+) and duration of the intervention (3–12 weeks) which is in line with previous meta-analyses (Pearson and Smart 2017; Cavalcante et al. 2019). Moreover, MICON exercise apart from the beneficial effects on circulating EPCs showed to enhance the functional capacity of MACs in CHF patients as well (Sarto et al. 2007; Erbs et al. 2010). In ACS patients, a structured MICON programme as short as 4 weeks can increase circulating EPCs irrespective of EPC phenotype, which is accompanied by improvement in \(V{\text{O}}_{{2{\text{peak}}}}\) and reduction in high sensitivity C-reactive protein (Cesari et al. 2013).
In PAD patients there were contrasting findings with two trials reporting an increase (Sandri et al. 2005; Schlager et al. 2011), one a reduction (Dopheide et al. 2016b) and another one no change (Sandri et al. 2005) in circulating EPCs. In the trials that reported increases in EPC levels, disease severity was higher than the two trials that did not find positive changes in EPCs as indicated by the baseline maximum walking distances: median values of 148 m (Sandri et al. 2005) and 101.5 m (Schlager et al. 2011) vs 401 m (Dopheide et al. 2016b) and 335 m (Sandri et al. 2005). A cross-sectional trial found that CD34+/VEGFR2+/CD45dim EPCs were proportionally higher in patients with increased disease severity (but not critical limb ischaemia CLI) (Dopheide et al. 2016a). On this basis, we propose that PAD patients with greater disease severity (but not CLI) would benefit from MICON exercise programmes due to the ongoing demand for endothelial repair and the augmented EPC response to exercise training.
In CAD patients the effects of a MICON exercise programme are inconclusive with results suggesting that a 4-week programme is not adequate to increase circulating EPCs (Sandri et al. 2005). It is worth noting that in this study, exercise was completed in bouts of 10 min repeated 6 times a day at an intensity of 70% HRpeak. Longer training programmes lasting 12 weeks showed a significant increase in EPC levels, however, this was not paralleled with significant improvement in FMD (Steiner et al. 2005; Paul et al. 2007). In contrast, 12 weeks of a MICON exercise programme increased FMD but not circulating EPCs (Van Craenenbroeck et al. 2015). These variations in findings can partly be explained by individual responses (responders and non-responders) to the given exercise stimulus. Paul et al. (2007) reported that despite the significant increase in circulating EPCs, 46 participants (23.9% of total of sample size) did not show any changes in EPC levels. They found no differences in baseline EPCs, anthropometric characteristics, medical therapy, or other potential parameters that would explain differences in EPC levels between responders and non-responders. This requires further investigation to ascertain the optimal dose of exercise to each patient. Another possible confounder is the (often not reported) training fidelity. In other words, it is assumed (but not monitored) that participants follow the training instructions they have received, in terms of frequency, duration and intensity of exercise, for the duration of the study (Ibeggazene et al. 2020).
Interventions combining resistance with MICON exercise produce variable results regarding EPC responses. HFrEF patients seem to benefit from such type of interventions as evidenced by improvements in FMD (Van Craenenbroeck et al. 2010b). In CAD patients only one trial found increases in EPC levels after 28 days of training (Laufs et al. 2004). In other studies, training for six (Hansen et al. 2011) and eight (Luk et al. 2012) weeks was not accompanied by EPC increases, despite FMD improvement in the latter trial. Several methodological differences contribute to the variability in findings. For example, the two trials which reported improvements in EPC numbers (Laufs et al. 2004; Van Craenenbroeck et al. 2010b) had participants of a lower aerobic capacity at baseline and seem to have prescribed higher exercise doses than the studies which showed no effect (Hansen et al. 2011; Luk et al. 2012) though the lack of detailed descriptions of the exercise interventions makes this hard to quantify. Nevertheless, both volume and intensity seem higher in these trials (Laufs et al. 2004; Van Craenenbroeck et al. 2010b). It is unfortunate that there seems to be a lack of detailed information regarding the resistance exercise prescription (Laufs et al. 2004; Luk et al. 2012), the exact time point of blood collection post-intervention (Laufs et al. 2004; Hansen et al. 2011; Luk et al. 2012) and whether the patients were fasted or not during the blood sampling (Laufs et al. 2004). Considering that EPC levels have been shown to peak at 24 h and remain elevated up to 48 h after an acute exercise bout in CAD patients (Adams et al. 2004), while consumption of beverages such as coffee and beer increase circulating EPCs in CAD and individuals with increased CVD risk respectively (Spyridopoulos et al. 2008; Chiva-Blanch et al. 2014), information about blood time collection and fasting status is essential.
When examining the effects of MICON exercise with the addition of callisthenics only one trial found that both CD34+/KDR+ and CD133+/KDR+ EPCs increased in patients with HFrEF irrespective of age (Sandri et al. 2016). The increased EPC numbers were accompanied by improvements in FMD, MACs migratory capacity towards SDF-1α, VEGF and SDF-1α. The lack of positive responses in another trial can be attributed to the short duration intervention (15 days) and the wide range of co-morbidities and cardiovascular risk factors within the cohort (Cesari et al. 2009). For example, cardiac patients with PAD had lower EPC numbers at baseline compared to the patients without, which probably contributed to a heterogeneity in terms of the effects of the exercise programme on EPC levels (Cesari et al. 2009). However, when the cohort was divided based on median percentage improvement in the six-minute walk test (6MWT), the patients with > 23% improvement had significantly increased EPC levels irrespective of the EPC phenotype (CD34+/KDR+; CD133+/KDR+; CD34+/CD133+/KDR+) (Cesari et al. 2009). Also, in the same patients, there was a positive relationship between EPC numbers and VEGF whereas in those with < 23% improvement in 6MWT there was a negative correlation between CRP and EPC levels. Moreover, in another trial, CAD patients who combined aerobic activities with callisthenics, despite a lack of changes in EPC numbers, there was an inverse relationship between circulating EPCs and VEGF (r = – 0.57, P = 0.007) (Gagliardi et al. 2016). The authors attributed this relationship to the increased incorporation of EPCs into sites of endothelial injury leading to reduced levels in circulation (Gagliardi et al. 2016). The above data are inconclusive and further investigation is required regarding the value of adding callisthenics into cardiac rehabilitation programmes alongside aerobic exercise. Nevertheless, evidence suggests that breaks of sedentary time every 20 min with five calisthenic exercises compared to sitting induce an increase in brachial shear rate (Carter and Gladwell 2017). Moreover, a 12 week aerobic calisthenic exercise programme led to an improvement in lipid profile and increase in plasma NOx (Guzel et al. 2012). However, no study to our knowledge investigated the effects of breaking sedentary time on circulating EPC levels. Considering the critical role of increased shear stress and NOx on EPC mobilisation and homing (Obi et al. 2014; Ozuyaman et al. 2005) further investigation is warranted in the use of structured callisthenic exercise programmes in clinical populations.
Three trials investigated the chronic effects of HIIT programmes on EPC levels in comparison to either MICON or combined (HIIT plus resistance exercise) modalities. This follows with contrasting findings as one trial reported significant increases in CD34+/KDR+ EPCs in the HIIT group (Jo et al. 2020), also accompanied by greater FMD improvements (Jo et al. 2020), whereas the other trial found no effects on CD34+/KDR+/CD45dim EPCs despite both the HIIT and MICON protocols induced similar improvements in FMD (Van Craenenbroeck et al. 2015). Moreover, addition of resistance exercise to HIIT programmes elicited positive changes on circulating EPCs, which were accompanied by concomitant increases in VEGF and a reduction in CRP (Kourek et al. 2020a). One possible reason for the contrasting findings is the varied pathological status of the participants (hypertensive MetS, CHF and CAD), which can potentially influence to circulating EPC kinetics. Furthermore, two trials were single centre (Jo et al. 2020; Kourek et al. 2020a) whereas the third one was a multicentre trial (Van Craenenbroeck et al. 2015); this may have led to a potential variation in the assessment processes and application of training programmes between centres (Conraads et al. 2015).
In conclusion, strong evidence exists that patients with HFrEF and ACS benefit from a structured exercise programme and see significant increases in circulating EPCs. MICON exercise programmes seems to primarily benefit PAD patients with increased disease severity. In CAD patients the findings are equivocal with the data showing large variability in terms of EPC phenotypes, training characteristics, disease severity and medication prescription. In hypertensive MetS, a single trial showed that a HIIT intervention was superior to a MICON exercise intervention in terms of EPC mobilisation. Only two trials included in this review compared two different exercise protocols against each other. Therefore, more head-to-head RCTs are needed to compare the effects of different exercise protocols on EPC mobilisation. Finally, the chronic effects of different exercise modalities on HFpEF and T1DM and T2DM are yet to be determined. It is also important that studies improve their reporting of patient characteristics (especially in terms of anthropometry and cardiovascular fitness as well as clinical details and medications) and the reporting of details of the exercise interventions (frequency, intensity and duration of exercise as well as type and mode). Additionally, as exercise effects are specific to the above characteristics, an attempt should be made to monitor adherence to the prescribed exercise programme (exercise fidelity) and results should be analysed and presented based both on (a) intention to treat and (b) per protocol.
Mechanisms for exercise-induced EPC mobilisation
The mechanisms through which exercise mobilises circulating EPCs are not fully understood. VEGF is an important humoral factor that is upregulated in cells exposed to hypoxia or ischaemia via hypoxia-inducible transcription factor 1 alpha (Forsythe et al. 1996). Acutely, a symptom-limited maximal exercise test was shown to increase plasma VEGF levels in ischaemic CAD and PAD patients (Adams et al. 2004; Sandri et al. 2011). These findings were not observed in non-ischaemic CAD patients (Adams et al. 2004) suggesting that transient exercise-induced ischaemia is an important factor in increasing VEGF and consequently circulating EPCs. The lack of increases in other angiogenic factors such as GM-CSF, basic fibroblast growth factor and SDF-1α makes it difficult to fully understand the exact mechanisms responsible for exercise-induced EPC mobilisation after acute exercise in the clinical context.
More evidence exists regarding the mechanisms for EPC mobilisation after chronic exercise. Exercise interventions showed that an increase in EPC levels was accompanied by an increase in VEGF in HFrEF (Erbs et al. 2010; Sandri et al. 2016; Sarto et al. 2007; Kourek et al. 2020a), in CAD (Steiner et al. 2005) and ischaemic PAD (Sandri et al. 2005). This increase in VEGF was found to be positively correlated with EPC levels (r = 0.66, P < 0.05) (Sandri et al. 2005). Besides, Cesari et al. (2009) found that post-cardiac surgery, in patients who had a > 23% median improvement in 6MWT, VEGF levels were positively correlated with EPC levels. The above indicates that VEGF play a positive role in the mobilisation of EPC into the circulation.
SDF-1α is another putative factor involved in EPC mobilisation via the upregulation of chemokine receptor 4/Janus Kinase-2 signalling (Xia et al. 2012). Three trials reported an elevation in SDF-1α levels which coincided with an increase in circulating EPCs (Erbs et al. 2010; Sandri et al. 2016; Sarto et al. 2007). In contrast, two trials failed to find an increase in SDF-1α despite an increase in EPC levels in the exercise groups (Van Craenenbroeck et al. 2010b; Eleuteri et al. 2013). These findings can be attributed to the difference in disease severity of the HFrEF patients between the trials. SDF-1α levels tend to be elevated in more advanced CHF patients (higher New York Heart Association Classification classes) (Valgimigli et al. 2004). The trials that reported no increases in SDF-1α had patients classified as New York Heart Association Classification II, whereas trials which reported increases in SDF-1α had a large portion of their patients at New York Heart Association Classification class III. Therefore, an exercise intervention can possibly be more beneficial to HFrEF patients with more advanced disease by causing greater increases in SDF-1α to compensate for the diminished EPC levels. Finally, the discrepancy in the results could be accounted to differences in the methodological steps quantifying circulating SDF-1α. The latter is a chemokine ligand to CXCR4 membrane receptor (Thon 2014). CXCR4 expressed on megakaryocyte lineage including platelets (Wang et al. 1998). Incomplete or no removal of platelets during plasma processing could lead to misleading results on SDF-1α levels caused by platelets activation instead of the release to the circulation from the tissues.
NOx bioavailability also plays a crucial regarding EPC mobilisation. The importance of NOx bioavailability on EPCs via eNOS mRNA expression was documented by Laufs et al. (2004) in trained wild type mice. In the present review two trials found that increases in circulating EPCs paralleled increases in NOx levels in HFrEF and hypertensive MetS patients respectively (Steiner et al. 2005; Jo et al. 2020). The increases in NOx levels were strongly correlated with increases in circulating EPCs (r = 0.83, P < 0.01) (Steiner et al. 2005).
Moreover, decreased EPC levels have been attributed to pro-inflammatory markers such as TNF-α and CRP. TNF-α is known for its myelosuppressive effects on EPC numbers in HFrEF (Valgimigli et al. 2004). In the present review we present evidence that TNF-α levels can be reduced after chronic exercise, which is paralleled by an increase in EPC numbers (Erbs et al. 2010; Gatta et al. 2012). This increase was inversely correlated with TNF-α levels (r = – 0.788, P < 0.01) (Gatta et al. 2012). It has been shown that incubation of cultured EPCs with SB203580 (a p38-kinase inhibitor) led to increased EPC numbers and diminished the negative effects on them from TNF-α (Seeger et al. 2005). Therefore, chronic exercise, through a reduction of systemic TNF-α levels might act as an inhibitor to the deleterious effects of the latter on EPC numbers and survival. Increased CRP levels are known to increase production of reactive oxygen species in EPCs causing apoptosis and necrosis (Fujii et al. 2006) and inhibit EPC differentiation and function via reduction of EPC eNOS mRNA expression (Verma et al. 2004). Some trials showed that CRP levels are reduced after chronic exercise (Cesari et al. 2009, 2013; Dopheide et al. 2016b; Kourek et al. 2020a), while others not (Paul et al. 2007; Hansen et al. 2011; Luk et al. 2012; Eleuteri et al. 2013). However, Cesari et al. (2013) using a large sample of patients with ACS (n = 112) found that baseline hsCRP levels were a significant predictor of increased EPC levels after the cardiac rehabilitation programme.
Finally, asymmetric dimethylarginine is an endogenous NOx inhibitor, which causes endothelial dysfunction and serves as a surrogate marker in CV disease (Sibal et al. 2010). Plasma ADMA is found to be inversely correlated with CD34+/CD133+ progenitor cells, reduced EPC function in vitro and decreased EPC eNOS activity (Thum et al. 2005). In the present systematic review despite the limited evidence, one RCT showed that asymmetric dimethylarginine levels were decreased at 3 and 6 months of exercise training compared to the control group. This decrease in asymmetric dimethylarginine levels paralleled an increase in CD34+/KDR+/CD133+ EPCs (Schlager et al. 2011).
In most of the trials assessing the chronic effects of exercise, the increase in circulating EPCs has coincided with an improvement in aerobic capacity (e.g., \(V{\text{O}}_{{2{\text{peak}}}}\), 6MWT, maximal walking distance) (Laufs et al. 2004; Sarto et al. 2007; Erbs et al. 2010; Adams et al. 2013; Cesari et al. 2013; Eleuteri et al. 2013; Mezzani et al. 2013; Kourek et al. 2020a). However, the role of circulating EPCs in the improvement of aerobic capacity is not clear. A cross-sectional study also found that the haemodialysis patients with various co-morbidities who had higher exercise capacity had higher basal EPC counts (Manfredini et al. 2007). Another recent cohort trial found that CD34+ progenitors but not CD34+CD133+KDR+ EPCs were predictors of all-cause and CVD mortality in CVD patients with higher level of physical activity associated with higher CD34 + progenitors (Muggeridge et al. 2021). To explain any mechanistic link between EPC mobilisation and \(V{\text{O}}_{{2{\text{peak}}}}\), one trial did not find any relationship (Eleuteri et al. 2013), while two found a positive relationship in ACS and HFrEF patients respectively (Cesari et al. 2013; Mezzani et al. 2013). Mezzani et al. (2013) suggested EPCs possibly had a positive effect on \(V{\text{O}}_{{2{\text{peak}}}}\) by mediating changes in the altered microvasculature which led to an improvement in oxygen utilisation and aerobic power. Certainly, it is possible that improvement in both variables is the product of an appropriate exercise dose and, otherwise, mechanistically unrelated.
The evidence of the impact of EPCs on macrocirculation as assessed by FMD is currently limited. In the present review, some trials found that the observed increases in circulating EPCs were paralleled by improvement in FMD (Erbs et al. 2010; Van Craenenbroeck et al. 2010b; Eleuteri et al. 2013; Sandri et al. 2016). Steiner et al. (2005) found that the change of EPCs was strongly positively correlated with changes in FMD (r = 0.81, P < 0.01). In contrast, Paul et al. (2007) did not find improvement in FMD despite an increase in EPC numbers, whereas van Craenenbroeck et al.(2015) reported that HIIT and MICON exercise protocols elicited equal improvements in FMD without any changes in EPC numbers. Furthermore, Luk et al. (2009) demonstrated that in CAD patients increased habitual physical activity levels were significantly correlated only with FMD and not with circulating EPCs suggesting an improvement in endothelial function through other mechanisms and not related to EPCs. Given that circulating EPC numbers and FMD are independent predictors of cardiometabolic diseases (Meyer et al. 2005; Kunz et al. 2006; Guazzi et al. 2009; Sambataro et al. 2014; Koller et al. 2016) further research is required to investigate the potential link between them following an exercise programme. The potential mechanisms of exercise-induced upregulation of EPCs for endothelial repair based on the findings of the current review are illustrated in Fig. 2.
Exercise training induces increases through shear stress to eNOS and consequently to nitric oxide bioavailability. In addition, exercise training increases the levels of pro-angiogenic factors such as VEGF and SDF-1α. EPCs mobilised from the bone marrow niche to the circulation following a gradient of SDF-1α. Long-term exercise leads to reduction to NO inhibitor, ADMA and pro-inflammatory markers such as TNF-α and CRP and consequently increase EPC proliferation and survival. The changes induced by exercise training in EPC levels coincide with improvements in endothelial function
Finally, the exercise-induced EPC mobilisation and the relationship with microcirculation is understudied. Patients with CAD and DM are characterised by altered microvascular function (Borges et al. 2016; Strain and Paldánius 2018),while in CHF patients impaired microcirculation is associated with exercise intolerance (Manetos et al. 2011). In the present systematic review, only a single trial assessed and found that a moderate exercise training intervention led to concomitant improvement in peripheral microcirculation and circulating EPCs in CHF patients (Mezzani et al. 2013), which possibly suggest that circulating EPCs play a healing role to the injured endothelium at the microcirculatory level. More research is warranted about the link between circulating EPCs and microcirculation during exercise interventions that implement different modalities, intensities and durations given that combined (resistance and aerobic) exercise or increased exercise frequency enhances microcirculation in CAD and MetS patients respectively (Borges et al. 2018; Marini et al. 2019).
Strengths and limitations
This systematic review has several strengths: To our knowledge, this is the first systematic review examining the acute effects along with the chronic effects of different exercise modalities in patients with cardiovascular and metabolic diseases. Previous systematic reviews and meta-analyses focussed only on the chronic effects of physical exercise on circulating EPCs in cardiovascular disease (Pearson and Smart 2017; Cavalcante et al. 2019; Ribeiro et al. 2013). However, they have been either contacted up to 2013 (Ribeiro et al. 2013) or the meta-analysis was based only on CHF patients which included only three trials (Pearson and Smart 2017). In addition, a recent meta-analysis (Cavalcante et al. 2019) focussing on effects of exercise on EPCs in CV disease failed to include two papers that included four RCTs (Sandri et al. 2005; Erbs et al. 2010). In the present systematic review, there was a rigorous application of the systematic review methodology and an extensive searching of the relevant literature. Despite that we did not included only RCTs we used valid quality assessment tools suitable for each study design. Moreover, we provide a detailed information of the different exercise modalities and the related angiogenic factors to delineate the existing literature related to EPC mobilisation in the peripheral blood and the potential exercise-induced mechanisms.
Our study has also some limitations. A limitation of the evidence in this review is the large variability in terms of the methodological assessment used by trials for identification of circulating EPCs. Two main methods have been used to identify EPCs: (a) flow cytometry based on combination of different cell surface markers, (b) in vitro cell culture methods (Medina et al. 2012). In culture, there are two distinct cell populations that differ based on their lineage, characteristics, and progeny. These are MACs and endothelial colony forming cells. In the present systematic review we identified, reported and used the most contemporary nomenclature for MACs, which are not progenitors but angiogenic cells that assist indirectly to vascular repair (Medina et al. 2017). However, it has been questioned whether cultured MACs really do exist in-vivo (Medina et al. 2017; Fadini et al. 2012). In addition, flow cytometry is the most appropriate method to quantify circulating EPCs (Fadini et al. 2008a; Van Craenenbroeck et al. 2013). For the reasons mentioned above we included only the trials that quantified circulating EPCs by flow cytometry as their primary method to provide a more consistent picture in the topic. However, despite flow cytometry being considered the gold standard to quantify EPCs in the blood, there is no standard phenotype that best represents this population (Madonna and De Caterina 2015). In the present review, we identified eleven different EPC phenotypes for the acute trials and eight for the chronic trials which confirm the large heterogeneity between the trials. However, CD34+/KDR+ or CD34+/KDR+/CD45dim antibody combination is the best compromise to quantify EPCs in terms of sensitivity, specificity and reliability in the clinical setting (Schmidt-Lucke et al. 2010; Fadini et al. 2012; Van Craenenbroeck et al. 2013). Differences in gating strategies to identify EPCs account to the variability in the present results. Van Craenenbroeck et al. (2008) found moderate to poor agreement between six different flow cytometric gating strategies for EPC quantification. Finally, flow cytometry related analytical factors possibly can explain part of the variability on findings within the same clinical population since the volume of blood samples, storage protocol, anti-coagulant tube selection, a wash step or not for red blood cell lysis and units of EPC expression are essential for reliable and accurate EPC enumeration (Hoymans et al. 2012; Van Craenenbroeck et al. 2013; Zhou et al. 2018).
In the present systematic review, the majority of the trials that investigated the acute effects in EPCs used post-exercise one-time point only for blood sample with the most common being 10 min post-exercise. This potentially masked any increases on circulating EPCs since it has been shown that EPC levels remain elevated after 24 h in ischaemic CAD and PAD patients (Adams et al. 2004; Sandri et al. 2011). Because of the potential delayed acute effects of exercise, it is not known if there was an effect of the last training session in chronic trials since a large number of them did not specify when the sample was taken at the end of the intervention. Moreover, in many acute and chronic trials, the condition of the patients whether were fasted or not during the blood sampling was not clearly stated.
In the present systematic review, we have shown that most of the participants were males (78.5%). Given that circulating EPCs are regulated by sex hormones such as oestrogens (Fadini et al. 2008b) more investigation required in the female population to identify potential different responses to exercise in EPC mobilisation.
All the acute trials that included patients with CV conditions the exercise protocol employed was a symptom-limited maximal exercise test on a treadmill or cycle ergometer. However, this does not represent a real exercise training condition (Volaklis et al. 2013), while the short duration of the test is likely an inadequate stimulus to significantly increase circulation of EPCs. It would therefore be prudent that future trials employ “real life” exercise training protocols based on international guidelines and include several time points to capture the time course of EPC mobilisation.
A recurrent limitation in some of the chronic trials was the lack of information regarding exercise prescription. For example, in many trials there was not provided sufficient information regarding the duration, repetitions or intensity (Laufs et al. 2004; Steiner et al. 2005; Sandri et al. 2005, 2016; Paul et al. 2007; Cesari et al. 2009; Van Craenenbroeck et al. 2010b; Luk et al. 2012; Adams et al. 2013; Gagliardi et al. 2016).
HIIT and whole-body resistance exercise found to be understudied in the present review. For example, whole-body resistance exercise showed to increase circulating EPCs in healthy males and females respectively (Ross et al. 2014; Ribeiro et al. 2017). Moreover, groups of patients such as HFpEF, T1DM and T2DM were understudied in terms of the effects of exercise on circulating EPCs and the angiogenic factors, therefore future trials should focus on these populations.
Finally, one overlooked topic is the interaction between medication and exercise interventions. For example, due to the known beneficial effects of statins (Lee and Poh 2014), changes in statin dosage can hinders the investigation of the effects of exercise on circulating EPCs. In chronic trials with CAD patients only some (Laufs et al. 2004; Steiner et al. 2005; Paul et al. 2007; Luk et al. 2012) clearly stated that the medication did not change throughout the intervention. This observation warrants more investigation because it is not known if it can (at least in part) explain the differences in the results in this patient group.
Conclusions
This systematic review provides evidence that an acute exercise bout increases EPC numbers as well as cultured MACs in ischaemic and revascularized CAD patients and patients with PAD, where these increases peak at 24 h post-exercise. In contrast, HFpEF have an impaired ability to mobilise EPCs. In HFrEF, inconclusive findings suggest that maximal exercise does not appear to favour EPC mobilisation, despite an increase in MACs migratory capacity. However, the evidence comes only from the use of a maximal exercise test as the exercise stimulus. Some evidence exists that HIIT and MICON exercise modalities acutely enhance EPC numbers. In patients with metabolic diseases EPC mobilisation is blunted compared to healthy matched controls.
In chronic trials, we found evidence that HFrEF and ACS patients benefit from increases in circulating EPCs and MACs migratory capacity as a result of participation in MICON exercise programme alone or with the addition of resistance exercise or group session with callisthenics. These improvements were irrespective of the EPC phenotypes used. PAD patients with reduced physical capacity seem to benefit from a MICON exercise programme. In CAD patients, findings are equivocal possibly due to variation in EPC phenotypes, training characteristics, disease severity and medication. In hypertensive MetS patients, a HIIT protocol seems to be superior for EPC mobilisation and improvement in vascular function.
The effects of exercise on circulating EPCs are related to angiogenic factors such as VEGF, SDF-1α, increase in NOx bioavailability, reduction in inflammation and asymmetric dimethylarginine. The potential relationship with FMD and \(V{\text{O}}_{{2{\text{peak}}}}\) is yet to be determined.
Despite the abundance of literature around circulating EPCs and exercise further research is still required to investigate the feasibility and dose–response relationship of various “real life” exercise training protocols, and to assess the time course of EPC release in the circulation.
Finally, head-to-head RCTs are now required to examine and compare various exercise protocols such as HIIT and whole-body resistance exercise in patients with cardiovascular and metabolic disease using standardised methodologies for accurate, valid and reliable EPC enumeration.
Abbreviations
- 1RM:
-
One repetition maximum strength
- 6MWT:
-
Six minute walk test
- CAD:
-
Coronary artery disease
- CHF:
-
Chronic heart failure
- CPET:
-
Cardiopulmonary exercise test
- CRP:
-
C- reactive protein
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- eNOS:
-
Endothelial nitric oxide synthase
- EPCs:
-
Endothelial progenitor cells
- FMD:
-
Flow-mediated dilatation
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- HFpEF:
-
Heart failure preserved ejection fraction
- HFrEF:
-
Heart failure reduced ejection fraction
- HIIT:
-
High intensity interval training
- MACs:
-
Myeloid angiogenic cells
- MetS:
-
Metabolic syndrome
- MICON:
-
Moderate intensity continuous training
- MMP:
-
Matrix metalloproteinase
- non-RCTs:
-
Non-randomised controlled trials
- NOx:
-
Nitric oxide metabolites (Nitrite/Nitrate)
- PAD:
-
Peripheral arterial disease
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCT:
-
Randomised controlled trial
- SDF-1α:
-
Stromal cell derived factor 1 alpha
- T1DM:
-
Type one diabetes mellitus
- T2DM:
-
Type two diabetes mellitus
- TESTEX:
-
Tool for the assessment of study quality and reporting in exercise
- TIMP-1:
-
Tissue inhibitor of metalloproteinase 1
- TNF-a:
-
Tumour necrosis factor 1 alpha
- VEGF:
-
Vascular endothelial growth factor
References
Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R (2004) Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 24(4):684–690. https://doi.org/10.1161/01.ATV.0000124104.23702.a0
Adams V, Heiker JT, Hollriegel R, Beck EB, Woitek FJ, Erbs S, Bluher M, Stumvoll M, Beck-Sickinger AG, Schuler G, Linke A (2013) Adiponectin promotes the migration of circulating angiogenic cells through p38-mediated induction of the CXCR4 receptor. Int J Cardiol 167(5):2039–2046. https://doi.org/10.1016/j.ijcard.2012.05.056
Amato M, Frigerio B, Castelnuovo S, Ravani A, Sansaro D, Tremoli E, Squellerio I, Cavalca V, Veglia F, Sirtori CR, Werba JP, Baldassarre D (2013) Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis 228(1):153–160. https://doi.org/10.1016/j.atherosclerosis.2013.02.037
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Asahara T, Kawamoto A, Masuda H (2011) Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells 29(11):1650–1655. https://doi.org/10.1002/stem.745
Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS (2017) Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med 51(6):494–503. https://doi.org/10.1136/bjsports-2015-095841
Black MA, Cable NT, Thijssen DH, Green DJ (2009) Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297(3):H1109-1116. https://doi.org/10.1152/ajpheart.00226.2009
Borges JP, Lopes GO, Verri V, Coelho MP, Nascimento PM, Kopiler DA, Tibirica E (2016) A novel effective method for the assessment of microvascular function in male patients with coronary artery disease: a pilot study using laser speckle contrast imaging. Braz J Med Biol Res 49(10):e5541. https://doi.org/10.1590/1414-431X20165541
Borges JP, Nascimento AR, Lopes GO, Medeiros-Lima DJM, Coelho MP, Nascimento PMC, Kopiler DA, Matsuura C, Mediano MFF, Tibirica E (2018) The impact of exercise frequency upon microvascular endothelium function and oxidative stress among patients with coronary artery disease. Clin Physiol Funct Imaging 38(5):840–846. https://doi.org/10.1111/cpf.12492
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, Welch V, Thomson H (2020) Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 368:l6890. https://doi.org/10.1136/bmj.l6890
Carter SE, Gladwell VF (2017) Effect of breaking up sedentary time with callisthenics on endothelial function. J Sports Sci 35(15):1508–1514. https://doi.org/10.1080/02640414.2016.1223331
Cavalcante SL, Lopes S, Bohn L, Cavero-Redondo I, Alvarez-Bueno C, Viamonte S, Santos M, Oliveira J, Ribeiro F (2019) Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol 38(11):817–827. https://doi.org/10.1016/j.repc.2019.02.016
Cesari F, Sofi F, Caporale R, Capalbo A, Marcucci R, Macchi C, Lova RM, Cellai T, Vannucci M, Gensini GF, Abbate R, Gori AM (2009) Relationship between exercise capacity, endothelial progenitor cells and cytochemokines in patients undergoing cardiac rehabilitation. Thromb Haemost 101(3):521–526
Cesari F, Marcucci R, Gori AM, Burgisser C, Francini S, Sofi F, Gensini GF, Abbate R, Fattirolli F (2013) Impact of a cardiac rehabilitation program and inflammatory state on endothelial progenitor cells in acute coronary syndrome patients. Int J Cardiol 167(5):1854–1859. https://doi.org/10.1016/j.ijcard.2012.04.157
Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW (2013) The role of immune-related myeloid cells in angiogenesis. Immunobiology 218(11):1370–1375. https://doi.org/10.1016/j.imbio.2013.06.010
Chen TG, Zhong ZY, Sun GF, Zhou YX, Zhao Y (2011) Effects of tumour necrosis factor-alpha on activity and nitric oxide synthase of endothelial progenitor cells from peripheral blood. Cell Prolif 44(4):352–359. https://doi.org/10.1111/j.1365-2184.2011.00764.x
Chiva-Blanch G, Condines, Magraner E, Roth I, Valderas-Martinez P, Arranz S, Casas R, Martinez-Huelamo M, Vallverdu-Queralt A, Quifer-Rada P, Lamuela-Raventos RM, Estruch R (2014) The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: a randomized clinical trial. Atherosclerosis 233(2):518–524. https://doi.org/10.1016/j.atherosclerosis.2013.12.048
Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, Denollet J, Frederix G, Goetschalckx K, Hoymans VY, Possemiers N, Schepers D, Shivalkar B, Voigt JU, Van Craenenbroeck EM, Vanhees L (2015) Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol 179:203–210. https://doi.org/10.1016/j.ijcard.2014.10.155
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa-Uva M, Valensi P, Wheeler DC, Group ESCSD (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486
De Biase C, De Rosa R, Luciano R, De Luca S, Capuano E, Trimarco B, Galasso G (2013) Effects of physical activity on endothelial progenitor cells (EPCs). Front Physiol 4:414. https://doi.org/10.3389/fphys.2013.00414
Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I (2006) Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55(3):774–779. https://doi.org/10.2337/diabetes.55.03.06.db05-1417
Dopheide JF, Geissler P, Rubrech J, Trumpp A, Zeller GC, Bock K, Dorweiler B, Dunschede F, Munzel T, Radsak MP, Espinola-Klein C (2016a) Inflammation is associated with a reduced number of pro-angiogenic Tie-2 monocytes and endothelial progenitor cells in patients with critical limb ischemia. Angiogenesis 19(1):67–78. https://doi.org/10.1007/s10456-015-9489-y
Dopheide JF, Geissler P, Rubrech J, Trumpp A, Zeller GC, Daiber A, Munzel T, Radsak MP, Espinola-Klein C (2016b) Influence of exercise training on proangiogenic TIE-2 monocytes and circulating angiogenic cells in patients with peripheral arterial disease. Clin Res Cardiol 105(8):666–676. https://doi.org/10.1007/s00392-016-0966-0
Eleuteri E, Mezzani A, Di Stefano A, Vallese D, Gnemmi I, Delle Donne L, Taddeo A, Della Bella S, Giannuzzi P (2013) Aerobic training and angiogenesis activation in patients with stable chronic heart failure: a preliminary report. Biomarkers 18(5):418–424. https://doi.org/10.3109/1354750X.2013.805342
Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, Mobius-Winkler S, Sandri M, Krankel N, Hambrecht R, Schuler G (2010) Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail 3(4):486–494. https://doi.org/10.1161/CIRCHEARTFAILURE.109.868992
Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A (2008a) Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis 197(2):496–503. https://doi.org/10.1016/j.atherosclerosis.2007.12.039
Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A (2008b) Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol 28(5):997–1004. https://doi.org/10.1161/ATVBAHA.107.159558
Fadini GP, Losordo D, Dimmeler S (2012) Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110(4):624–637. https://doi.org/10.1161/circresaha.111.243386
Fernandez JM, Rosado-Alvarez D, Da Silva Grigoletto ME, Rangel-Zuniga OA, Landaeta-Diaz LL, Caballero-Villarraso J, Lopez-Miranda J, Perez-Jimenez F, Fuentes-Jimenez F (2012) Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin Sci (lond) 123(6):361–373. https://doi.org/10.1042/CS20110477
Fornoni A, Raij L (2005) Metabolic syndrome and endothelial dysfunction. Curr Hypertens Rep 7(2):88–95
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16(9):4604–4613. https://doi.org/10.1128/mcb.16.9.4604
Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S (2006) C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol 26(11):2476–2482. https://doi.org/10.1161/01.ATV.0000242794.65541.02
Gagliardi JA, Maciel N, Castellano JL, Masoli O, Miksztowicz V, Berg G, Bermejo E, Lazzari M, Gelpi RJ (2016) Relationship between endothelial progenitor cells and vascular endothelial growth factor and its variation with exercise. Thromb Res 137:92–96. https://doi.org/10.1016/j.thromres.2015.11.012
Gatta L, Armani A, Iellamo F, Consoli C, Molinari F, Caminiti G, Volterrani M, Rosano GM (2012) Effects of a short-term exercise training on serum factors involved in ventricular remodelling in chronic heart failure patients. Int J Cardiol 155(3):409–413. https://doi.org/10.1016/j.ijcard.2010.10.045
Gevaert AB, Beckers PJ, Van Craenenbroeck AH, Lemmens K, Van De Heyning CM, Heidbuchel H, Vrints CJ, Van Craenenbroeck EM (2019) Endothelial dysfunction and cellular repair in heart failure with preserved ejection fraction: response to a single maximal exercise bout. Eur J Heart Fail 21(1):125–127. https://doi.org/10.1002/ejhf.1339
Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA (2003) Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41(10):1769–1775
Goncalves FM, Jacob-Ferreira AL, Gomes VA, Casella-Filho A, Chagas AC, Marcaccini AM, Gerlach RF, Tanus-Santos JE (2009) Increased circulating levels of matrix metalloproteinase (MMP)-8, MMP-9, and pro-inflammatory markers in patients with metabolic syndrome. Clin Chim Acta 403(1–2):173–177. https://doi.org/10.1016/j.cca.2009.02.013
Guazzi M, Reina G, Gripari P, Tumminello G, Vicenzi M, Arena R (2009) Prognostic value of flow-mediated dilatation following myocardial infarction. Int J Cardiol 132(1):45–50. https://doi.org/10.1016/j.ijcard.2007.10.036
Guzel NA, Pinar L, Colakoglu F, Karacan S, Ozer C (2012) Long-term callisthenic exercise-related changes in blood lipids, homocysteine, nitric oxide levels and body composition in middle-aged healthy sedentary women. China J Physiol 55(3):202–209. https://doi.org/10.4077/CJP.2012.AMM122
Hadi HA, Suwaidi JA (2007) Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3(6):853–876
Hadi HA, Carr CS, Al Suwaidi J (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1(3):183–198
Hansen D, Eijnde BO, Roelants M, Broekmans T, Rummens JL, Hensen K, Daniels A, Van Erum M, Bonne K, Reyckers I, Alders T, Berger J, Dendale P (2011) Clinical benefits of the addition of lower extremity low-intensity resistance muscle training to early aerobic endurance training intervention in patients with coronary artery disease: a randomized controlled trial. J Rehabil Med 43(9):800–807. https://doi.org/10.2340/16501977-0853
Hortenhuber T, Rami-Mehar B, Satler M, Nagl K, Hobaus C, Hollerl F, Koppensteiner R, Schernthaner G, Schober E, Schernthaner GH (2013) Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes over time. Diabetes Care 36(6):1647–1653. https://doi.org/10.2337/dc12-1206
Hoymans VY, Van Craenenbroeck AH, Bruyndonckx L, van Ierssel SH, Vrints CJ, Conraads VM, Van Craenenbroeck EM (2012) TransFix(R) for delayed flow cytometry of endothelial progenitor cells and angiogenic T cells. Microvasc Res 84(3):384–386. https://doi.org/10.1016/j.mvr.2012.08.007
Hristov M, Erl W, Weber PC (2003) Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 23(7):1185–1189. https://doi.org/10.1161/01.ATV.0000073832.49290.B5
Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, Lo WY, Wu TC, Sata M, Chen JW, Lin SJ (2009) Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 29(8):1179–1184. https://doi.org/10.1161/ATVBAHA.109.189175
Ibeggazene S, Moore C, Tsakirides C, Swainson M, Ispoglou T, Birch K (2020) UK cardiac rehabilitation fit for purpose? A community-based observational cohort study. BMJ Open 10(10):e037980. https://doi.org/10.1136/bmjopen-2020-037980
Jo E-A, Wu S-S, Han H-R, Park J-J, Park S, Cho K-I (2019) Effects of exergaming in postmenopausal women with high cardiovascular risk: a randomized controlled trial. Clin Cardiol. https://doi.org/10.1002/clc.23324
Jo E-A, Cho K-I, Park J-J, Im D-S, Choi J-H, Kim B-J (2020) Effects of high-intensity interval training versus moderate-intensity continuous training on epicardial fat thickness and endothelial function in hypertensive metabolic syndrome. Metab Syndr Relat Disord. https://doi.org/10.1089/met.2018.0128
Kazmierski M, Wojakowski W, Michalewska-Wludarczyk A, Podolecka E, Kotowski M, Machalinski B, Tendera M (2015) Exercise-induced mobilisation of endothelial progenitor cells in patients with premature coronary heart disease. Kardiol Pol 73(6):411–418. https://doi.org/10.5603/KP.a2014.0248
Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM (2007) Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol 49(24):2341–2349. https://doi.org/10.1016/j.jacc.2007.01.095
Koller L, Hohensinner P, Sulzgruber P, Blum S, Maurer G, Wojta J, Hulsmann M, Niessner A (2016) Prognostic relevance of circulating endothelial progenitor cells in patients with chronic heart failure. Thromb Haemost 116(2):309–316. https://doi.org/10.1160/TH16-01-0051
Kourek C, Alshamari M, Mitsiou G, Psarra K, Delis D, Linardatou V, Pittaras T, Ntalianis A, Papadopoulos C, Panagopoulou N, Vasileiadis I, Nanas S, Karatzanos E (2020a) The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: comparing two different exercise training protocols. Int J Cardiol Heart Vasc 32:100702. https://doi.org/10.1016/j.ijcha.2020.100702
Kourek C, Karatzanos E, Psarra K, Georgiopoulos G, Delis D, Linardatou V, Gavrielatos G, Papadopoulos C, Nanas S, Dimopoulos S (2020b) Endothelial progenitor cells mobilization after maximal exercise according to heart failure severity. World J Cardiol 12(11):526–539. https://doi.org/10.4330/wjc.v12.i11.526
Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S (2012) Circulating endothelial and progenitor cells: Evidence from acute and long-term exercise effects. World J Cardiol 4(12):312–326. https://doi.org/10.4330/wjc.v4.i12.312
Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED (2006) Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 152(1):190–195. https://doi.org/10.1016/j.ahj.2006.02.001
Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G (2004) Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109(2):220–226. https://doi.org/10.1161/01.CIR.0000109141.48980.37
Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Bohm M, Kindermann W, Nickenig G (2005) Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil 12(4):407–414
Lee PS, Poh KK (2014) Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells 6(3):355–366. https://doi.org/10.4252/wjsc.v6.i3.355
Lu CL, Leu JG, Liu WC, Zheng CM, Lin YF, Shyu JF, Wu CC, Lu KC (2016) Endothelial progenitor cells predict long-term mortality in hemodialysis patients. Int J Med Sci 13(3):240–247. https://doi.org/10.7150/ijms.14209
Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Fong DY, Lee SW, Li SW, Tam S, Lau CP, Tse HF (2009) Habitual physical activity is associated with endothelial function and endothelial progenitor cells in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 16(4):464–471. https://doi.org/10.1097/HJR.0b013e32832b38be
Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Lee SW, Li SW, Fong B, Wong WK, Tam S, Lau CP, Tse HF (2012) Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol 19(4):830–839. https://doi.org/10.1177/1741826711415679
Lutz AH, Blumenthal JB, Landers-Ramos RQ, Prior SJ (2016) Exercise-induced endothelial progenitor cell mobilization is attenuated in impaired glucose tolerance and type 2 diabetes. J Appl Physiol (1985) 121(1):36–41. https://doi.org/10.1152/japplphysiol.00349.2016
Madonna R, De Caterina R (2015) Circulating endothelial progenitor cells: do they live up to their name? Vascul Pharmacol 67–69:2–5. https://doi.org/10.1016/j.vph.2015.02.018
Maiorino MI, Bellastella G, Petrizzo M, Gicchino M, Caputo M, Giugliano D, Esposito K (2017) Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: follow-up of a randomized trial. Eur J Prev Cardiol 24(4):399–408. https://doi.org/10.1177/2047487316676133
Manetos C, Dimopoulos S, Tzanis G, Vakrou S, Tasoulis A, Kapelios C, Agapitou V, Ntalianis A, Terrovitis J, Nanas S (2011) Skeletal muscle microcirculatory abnormalities are associated with exercise intolerance, ventilatory inefficiency, and impaired autonomic control in heart failure. J Heart Lung Transplant 30(12):1403–1408. https://doi.org/10.1016/j.healun.2011.08.020
Manfredini F, Rigolin GM, Malagoni AM, Soffritti S, Boari B, Conconi F, Castoldi GL, Catizone L, Zamboni P, Manfredini R (2007) Exercise capacity and circulating endothelial progenitor cells in hemodialysis patients. Int J Sports Med 28(5):368–373. https://doi.org/10.1055/s-2006-924363
Marini E, Mariani PG, Ministrini S, Pippi R, Aiello C, Reginato E, Siepi D, Innocente S, Lombardini R, Paltriccia R, Kararoudi MN, Lupattelli G, De Feo P, Pasqualini L (2019) Combined aerobic and resistance training improves microcirculation in metabolic syndrome. J Sports Med Phys Fitness 59(9):1571–1576. https://doi.org/10.23736/s0022-4707.18.09077-1
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 42(36):3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW (2011) Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med (cambridge, Mass) 17(9–10):1045–1055. https://doi.org/10.2119/molmed.2011.00129
Medina RJ, O’Neill CL, O’Doherty TM, Wilson SE, Stitt AW (2012) Endothelial progenitors as tools to study vascular disease. Stem Cells Int 2012:346735. https://doi.org/10.1155/2012/346735
Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW (2017) Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 6(5):1316–1320. https://doi.org/10.1002/sctm.16-0360
Meyer B, Mortl D, Strecker K, Hulsmann M, Kulemann V, Neunteufl T, Pacher R, Berger R (2005) Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B-type natriuretic peptide. J Am Coll Cardiol 46(6):1011–1018. https://doi.org/10.1016/j.jacc.2005.04.060
Mezzani A, Grassi B, Jones AM, Giordano A, Corra U, Porcelli S, Della Bella S, Taddeo A, Giannuzzi P (2013) Speeding of pulmonary VO2 on-kinetics by light-to-moderate-intensity aerobic exercise training in chronic heart failure: clinical and pathophysiological correlates. Int J Cardiol 167(5):2189–2195. https://doi.org/10.1016/j.ijcard.2012.05.124
Mitsiou G, Karatzanos E, Smilios I, Psarra K, Patsaki I, Douda HT, Ntalianis A, Nanas S, Tokmakidis SP (2020) Exercise promotes endothelial progenitor cell mobilization in patients with chronic heart failure. Eur J Prev Cardiol. https://doi.org/10.1093/eurjpc/zwaa046
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Muggeridge D, Dodd J, Ross MD (2021) CD34(+) progenitors are predictive of mortality and are associated with physical activity in cardiovascular disease patients. Atherosclerosis 333:108–115. https://doi.org/10.1016/j.atherosclerosis.2021.07.004
National Heart LaBI National Heart, Lung and Blood Institute, Study Quality Assesment Tools. National Heart, Lung and Blood Institute (NHLBI) (2021) https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed March 2020
Obi S, Yamamoto K, Ando J (2014) Effects of shear stress on endothelial progenitor cells. J Biomed Nanotechnol 10(10):2586–2597. https://doi.org/10.1166/jbn.2014.2014
Ozuyaman B, Ebner P, Niesler U, Ziemann J, Kleinbongard P, Jax T, Godecke A, Kelm M, Kalka C (2005) Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost 94(4):770–772. https://doi.org/10.1160/TH05-01-0038
Palmefors H, DuttaRoy S, Rundqvist B, Borjesson M (2014) The effect of physical activity or exercise on key biomarkers in atherosclerosis—a systematic review. Atherosclerosis 235(1):150–161. https://doi.org/10.1016/j.atherosclerosis.2014.04.026
Paul JD, Powell TM, Thompson M, Benjamin M, Rodrigo M, Carlow A, Annavajjhala V, Shiva S, Dejam A, Gladwin MT, McCoy JP, Zalos G, Press B, Murphy M, Hill JM, Csako G, Waclawiw MA, Cannon RO 3rd (2007) Endothelial progenitor cell mobilization and increased intravascular nitric oxide in patients undergoing cardiac rehabilitation. J Cardiopulm Rehabil Prev 27(2):65–73. https://doi.org/10.1097/01.HCR.0000265031.10145.50
Pearson MJ, Smart NA (2017) Effect of exercise training on endothelial function in heart failure patients: a systematic review meta-analysis. Int J Cardiol 231:234–243. https://doi.org/10.1016/j.ijcard.2016.12.145
Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS (2015) The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 45(5):679–692. https://doi.org/10.1007/s40279-015-0321-z
Ribeiro F, Ribeiro IP, Alves AJ, do Ceu Monteiro M, Oliveira NL, Oliveira J, Amado F, Remiao F, Duarte JA (2013) Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil 92(11):1020–1030. https://doi.org/10.1097/PHM.0b013e31829b4c4f
Ribeiro F, Ribeiro IP, Goncalves AC, Alves AJ, Melo E, Fernandes R, Costa R, Sarmento-Ribeiro AB, Duarte JA, Carreira IM, Witkowski S, Oliveira J (2017) Effects of resistance exercise on endothelial progenitor cell mobilization in women. Sci Rep 7(1):17880. https://doi.org/10.1038/s41598-017-18156-6
Rocha NG, Sales AR, Penedo LA, Pereira FS, Silva MS, Miranda RL, Silva JF, Silva BM, Santos AA, Nobrega AC (2015) Impaired Circulating Angiogenic Cells Mobilization and Metalloproteinase-9 Activity after Dynamic Exercise in Early Metabolic Syndrome. Biomed Res Int 2015:920356. https://doi.org/10.1155/2015/920356
Ross MD, Wekesa AL, Phelan JP, Harrison M (2014) Resistance exercise increases endothelial progenitor cells and angiogenic factors. Med Sci Sports Exerc 46(1):16–23. https://doi.org/10.1249/MSS.0b013e3182a142da
Rummens JL, Daniels A, Dendale P, Hensen K, Hendrikx M, Berger J, Koninckx R, Hansen D (2012) Suppressed increase in blood endothelial progenitor cell content as result of single exhaustive exercise bout in male revascularised coronary artery disease patients. Acta Clin Belg 67(4):262–269. https://doi.org/10.2143/ACB.67.4.2062670
Sambataro M, Seganfreddo E, Canal F, Furlan A, Del Pup L, Niero M, Paccagnella A, Gherlinzoni F, Dei Tos AP (2014) Prognostic significance of circulating and endothelial progenitor cell markers in type 2 diabetic foot. Int J Vasc Med 2014:589412. https://doi.org/10.1155/2014/589412
Sandri M, Adams V, Gielen S, Linke A, Lenk K, Krankel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G, Hambrecht R (2005) Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation 111(25):3391–3399. https://doi.org/10.1161/CIRCULATIONAHA.104.527135
Sandri M, Beck EB, Adams V, Gielen S, Lenk K, Hollriegel R, Mangner N, Linke A, Erbs S, Mobius-Winkler S, Scheinert D, Hambrecht R, Schuler G (2011) Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil 18(1):55–64. https://doi.org/10.1097/HJR.0b013e32833ba654
Sandri M, Viehmann M, Adams V, Rabald K, Mangner N, Hollriegel R, Lurz P, Erbs S, Linke A, Kirsch K, Mobius-Winkler S, Thiery J, Teupser D, Hambrecht R, Schuler G, Gielen S (2016) Chronic heart failure and aging - effects of exercise training on endothelial function and mechanisms of endothelial regeneration: Results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur J Prev Cardiol 23(4):349–358. https://doi.org/10.1177/2047487315588391
Sarto P, Balducci E, Balconi G, Fiordaliso F, Merlo L, Tuzzato G, Pappagallo GL, Frigato N, Zanocco A, Forestieri C, Azzarello G, Mazzucco A, Valenti MT, Alborino F, Noventa D, Vinante O, Pascotto P, Sartore S, Dejana E, Latini R (2007) Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail 13(9):701–708. https://doi.org/10.1016/j.cardfail.2007.06.722
Scalone G, De Caterina A, Leone AM, Tritarelli A, Mollo R, Pinnacchio G, D’Amario D, Lanza GA, Crea F (2013) Effect of exercise on circulating endothelial progenitor cells in microvascular angina. Circ J 77(7):1777–1782
Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S (2011) Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 217(1):240–248. https://doi.org/10.1016/j.atherosclerosis.2011.03.018
Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S (2010) Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS ONE 5(11):e13790. https://doi.org/10.1371/journal.pone.0013790
Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S (2005) p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation 111(9):1184–1191. https://doi.org/10.1161/01.CIR.0000157156.85397.A1
Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N (2018) Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol 9:2187. https://doi.org/10.3389/fimmu.2018.02187
Sen S, McDonald SP, Coates PT, Bonder CS (2011) Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (lond) 120(7):263–283. https://doi.org/10.1042/CS20100429
Shaffer RG, Greene S, Arshi A, Supple G, Bantly A, Moore JS, Parmacek MS, Mohler ER 3rd (2006) Effect of acute exercise on endothelial progenitor cells in patients with peripheral arterial disease. Vasc Med 11(4):219–226. https://doi.org/10.1177/1358863x06072213
Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103(23):2776–2779
Sibal L, Agarwal SC, Home PD, Boger RH (2010) The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6(2):82–90. https://doi.org/10.2174/157340310791162659
Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C, Cornelissen V, Dieberg G (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13(1):9–18. https://doi.org/10.1097/XEB.0000000000000020
Spyridopoulos I, Fichtlscherer S, Popp R, Toennes SW, Fisslthaler B, Trepels T, Zernecke A, Liehn EA, Weber C, Zeiher AM, Dimmeler S, Haendeler J (2008) Caffeine enhances endothelial repair by an AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol 28(11):1967–1974. https://doi.org/10.1161/ATVBAHA.108.174060
Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD (1996) Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97(11):2601–2610. https://doi.org/10.1172/JCI118709
Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, Penka M, Wolzt M, Huber K, Wojta J, Minar E, Kopp CW (2005) Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis 181(2):305–310. https://doi.org/10.1016/j.atherosclerosis.2005.01.006
Strain WD, Paldánius PM (2018) Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 17(1):57. https://doi.org/10.1186/s12933-018-0703-2
Thon JN (2014) SDF-1 directs megakaryocyte relocation. Blood 124(2):161–163. https://doi.org/10.1182/blood-2014-05-571653
Thum T, Tsikas D, Stein S, Schultheiss M, Eigenthaler M, Anker SD, Poole-Wilson PA, Ertl G, Bauersachs J (2005) Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol 46(9):1693–1701. https://doi.org/10.1016/j.jacc.2005.04.066
Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R (2004) CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 110(10):1209–1212. https://doi.org/10.1161/01.CIR.0000136813.89036.21
Van Craenenbroeck EM, Conraads VM, Van Bockstaele DR, Haine SE, Vermeulen K, Van Tendeloo VF, Vrints CJ, Hoymans VY (2008) Quantification of circulating endothelial progenitor cells: a methodological comparison of six flow cytometric approaches. J Immunol Methods 332(1–2):31–40. https://doi.org/10.1016/j.jim.2007.12.006
Van Craenenbroeck EM, Denollet J, Paelinck BP, Beckers P, Possemiers N, Hoymans VY, Vrints CJ, Conraads VM (2009) Circulating CD34+/KDR+ endothelial progenitor cells are reduced in chronic heart failure patients as a function of Type D personality. Clin Sci (lond) 117(4):165–172. https://doi.org/10.1042/CS20080564
Van Craenenbroeck EM, Beckers PJ, Possemiers NM, Wuyts K, Frederix G, Hoymans VY, Wuyts F, Paelinck BP, Vrints CJ, Conraads VM (2010a) Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur Heart J 31(15):1924–1934. https://doi.org/10.1093/eurheartj/ehq058
Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, Vrints CJ, Conraads VM (2010b) Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol 105(5):665–676. https://doi.org/10.1007/s00395-010-0105-4
Van Craenenbroeck EM, Bruyndonckx L, Van Berckelaer C, Hoymans VY, Vrints CJ, Conraads VM (2011) The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur J Appl Physiol 111(9):2375–2379. https://doi.org/10.1007/s00421-011-1843-1
Van Craenenbroeck EM, Van Craenenbroeck AH, van Ierssel S, Bruyndonckx L, Hoymans VY, Vrints CJ, Conraads VM (2013) Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. Int J Cardiol 167(5):1688–1695. https://doi.org/10.1016/j.ijcard.2012.10.047
Van Craenenbroeck EM, Frederix G, Pattyn N, Beckers P, Van Craenenbroeck AH, Gevaert A, Possemiers N, Cornelissen V, Goetschalckx K, Vrints CJ, Vanhees L, Hoymans VY (2015) Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: a SAINTEX-CAD substudy. Am J Physiol Heart Circ Physiol 309(11):H1876-1882. https://doi.org/10.1152/ajpheart.00341.2015
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89(1):E1-7
Verma S, Anderson TJ (2002) Fundamentals of endothelial function for the clinical cardiologist. Circulation 105(5):546–549
Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ (2004) C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109(17):2058–2067. https://doi.org/10.1161/01.CIR.0000127577.63323.24
Volaklis KA, Tokmakidis SP, Halle M (2013) Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin Res Cardiol 102(4):249–257. https://doi.org/10.1007/s00392-012-0517-2
Waclawovsky G, Umpierre D, Figueira FR, De Lima ES, Alegretti AP, Schneider L, Matte US, Rodrigues TC, Schaan BD (2016) Exercise on progenitor cells in healthy subjects and patients with type 1 diabetes. Med Sci Sports Exerc 48(2):190–199. https://doi.org/10.1249/MSS.0000000000000764
Wang JF, Liu ZY, Groopman JE (1998) The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood 92(3):756–764
Werner N, Nickenig G (2006) Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol 26(2):257–266. https://doi.org/10.1161/01.ATV.0000198239.41189.5d
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353(10):999–1007. https://doi.org/10.1056/NEJMoa043814
West DJ, Campbell MD, Gonzalez JT, Walker M, Stevenson EJ, Ahmed FW, Wijaya S, Shaw JA, Weaver JU (2015) The inflammation, vascular repair and injury responses to exercise in fit males with and without Type 1 diabetes: an observational study. Cardiovasc Diabetol 14:71. https://doi.org/10.1186/s12933-015-0235-y
Widlansky ME, Gokce N, Keaney JF Jr, Vita JA (2003) The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42(7):1149–1160
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Xia WH, Li J, Su C, Yang Z, Chen L, Wu F, Zhang YY, Yu BB, Qiu YX, Wang SM, Tao J (2012) Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell 11(1):111–119. https://doi.org/10.1111/j.1474-9726.2011.00758.x
Zhou F, Zhou Y, Yang M, Wen J, Dong J, Tan W (2018) Optimized multiparametric flow cytometric analysis of circulating endothelial cells and their subpopulations in peripheral blood of patients with solid tumors: a technical analysis. Cancer Manag Res 10:447–464. https://doi.org/10.2147/CMAR.S157837
Author information
Authors and Affiliations
Contributions
Panagiotis Ferentinos, Costas Tsakirides, Michelle Swainson, Marrissa Martyn-St James, Adam Davison and Theocharis Ispoglou: Conceptualization. Panagiotis Ferentinos, Costas Tsakirides, Michelle Swainson, Marrissa Martyn-St James, Adam Davison and Theocharis Ispoglou: Methodology. Panagiotis Ferentinos, Costas Tsakirides, Michelle Swainson: Investigation and data curation. Panagiotis Ferentinos, Costas Tsakirides and Theocharis Ispoglou: Formal analysis.Panagiotis Ferentinos, Costas Tsakirides and Theocharis Ispoglou: Writing original draft. Panagiotis Ferentinos, Costas Tsakirides, Michelle Swainson, Marrissa Martyn-St James, Adam Davison and Theocharis Ispoglou: Writing-Reviewing and Editing.
Corresponding author
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferentinos, P., Tsakirides, C., Swainson, M. et al. The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur J Appl Physiol 122, 815–860 (2022). https://doi.org/10.1007/s00421-021-04876-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04876-1