Abstract
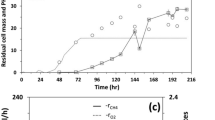
Slow growth and relatively low cell densities of methanotrophs have limited their uses in industrial applications. In this study, a novel method for rapid cultivation of Methylosinus trichosporium OB3b was studied by adding a water-immiscible organic solvent in the medium. Paraffin oil was the most effective at enhancing cell growth and final cell density. This is at least partially due to the increase of methane gas transfer between gas and medium phases since methane solubility is higher in paraffin than in water/nitrate minimal salt medium. During cultivation with paraffin oil at 5% (v/v) in the medium, M. trichosporium OB3b cells also showed higher concentrations of the intermediary metabolites, such as formic acid and pyruvic acid, and consumed more methane compared with the control. Paraffin as methane vector to improve methanotroph growth was further studied in a 5-L fermentor at three concentrations (i.e., 2.5%, 5%, and 10%). Cell density reached about 14 g dry weight per liter with 5% paraffin, around seven times higher than that of the control (without paraffin). Cells cultivated with paraffin tended to accumulate around the interface between oil droplets and the water phase and could exist in oil phase in the case of 10% (v/v) paraffin. These results indicated that paraffin could enhance methanotroph growth, which is potentially useful in cultivation of methanotrophs in large scale in industry.







Similar content being viewed by others
References
Arcangeli JP, Arvin E (1997) Modelling of the growth of a methanotrophic biofilm. Water Sci Technol 36:99–204
Borodina E, Nichol T, Dumont MG, Smith TJ, Murrell JC (2007) Mutagenesis of the “leucine gate” to explore the basis of catalytic versatility in soluble methane monooxygenase. Appl Environ Microbiol 73:6460–6467
Chu KH, Alvarez-Cohen L (1998) Effect of nitrogen source on growth and trichloroethylene degradation by methane-oxidizing bacteria. Appl Environ Microbiol 64:3451–3457
Clark TR, Roberto FF (1996) Methylosinus trichosporium OB3b whole cell methane monooxygenase activity in a biphasic matrix. Appl Environ Microbiol 45:658–663
Cornish A, McDonald J, Burrows KJ, King TS, Scott D, Higgins IJ (1985) Succinate as an in vitro electron donor for the particulate methane mono-oxygenase of Methylosinus trichosporium OB3b. Biotechnol Lett 7:319–324
Crolla A, Kennedy KJ (2001) Optimization of citric acid production from Candida lipolytica Y-1095 using n-paraffin. J Biotechnol 89:27–40
Crolla A, Kennedy KJ (2004) Fed-batch production of citric acid by Candida lipolytica grown on n-paraffins. J Biotechnol 110:73–84
Dalton H (2005) The Leeuwenhoek Lecture (2000) The natural and unnatural history of methane oxidizing bacteria. Philos Trans R Soc B 360:1207–1222
Dunfield PF, Liesack W, Henckel T, Knowles Rand Conrad R (1999) High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl Environ Microbiol 65:1009–1014
Galaction AI, Cascaval D, Oniscu C, Turnea M (2004) Enhancement of oxygen mass transfer in stirred bioreactors using oxygen-vectors. 1. Simulated fermentation broths. Bioprocess Biosyst Eng 26:231–238
Galaction AI, Cascaval D, Turnea M, Folescu E (2005) Enhancement of oxygen mass transfer in stirred bioreactors using oxygen-vectors 2. Propionibacterium shermanii broths. Bioprocess Biosyst Eng 27:263–271
Gou Z, Xing X-H, Luo M, Jiang H, Han B, Wu H, Wang L, Zhang F (2006) Functional expression of the particulate methane mono-oxygenase gene in recombinant Rhodococcus erythropolis. FEMS Microbiol Lett 263:136–41
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Helm J, Wendlandt KD, Rogge G, Kappelmeyer U (2006) Characterizing a stable methane-utilizing mixed culture used in the synthesis of a high-quality biopolymer in an open system. J Appl Microbiol 101:387–395
Hesselsoe M, Boysen S, Iversen N, Jorgensen L, Murrell JC, McDonald I, Radajewski S, Thestrup H, Roslev P (2005) Degradation of organic pollutants by methane grown microbial consortia. Biodegradation 16:435–448
Holmes AJ, Roslev P, McDonald IR, Iversen N, Henriksen K, Murrell JC (1999) Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol 65:3312–3318
Jitnuyanont P, Sayavedra-Soto LA, Semprini L (2001) Bioaugmentation of butane-utilizing microorganisms to promote cometabolism of 1,1,1-trichloroethane in groundwater microcosms. Biodegradation 12:11–22
Lai LS, Tsai TH, Wang TC (2002) Application of oxygen vectors to Aspergillus terreus cultivation. J Biosci Bioeng 94:453–459
Lee J, Soni BK, Kelley RL (1996) Cell growth and oxygen transfer in Methylosinus trichosporium OB3b cultures. Biotechnol Lett 18:903–908
Lee SG, Goo JH, Kim HG, Oh JI, Kim YM, Kim SW (2004) Optimization of methanol biosynthesis from methane using Methylosinus trichosporium OB3b. Biotechnol Lett 26:947–950
Lee SW, Keeney DR, Lim DH, Dispirito AA, Semrau JD (2006) Mixed pollutant degradation by Methylosinus trichosporium OB3b expressing either soluble or particulate methane monooxygenase: can the tortoise beat the hare? Appl Environ Microbiol 72:7503–7509
Lieberman RL, Rosenzweig AC (2004) Biological methane oxidation: regulation, biochemistry, and active site structure of particulate methane monooxygenase. Crit Rev Biochem Mol 39:147–164
Menge M, Mukherjee J, Scheper T (2001) Application of oxygen vectors to Claviceps purpurea cultivation. Appl Microbiol Biotechnol 55:411–416
Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Muller J, Lippard SJ (2001) Dioxygen activation and methane hydroxylation by soluble methane monooxygenase: a tale of two irons and three proteins. Angewandte Chemie–International Edition 40:2782–2807
Murrell JC, McDonald IR, Gilbert B (2000) Regulation of methane monooxygenase genes by copper ions. Trends Microbiol 8:221–225
Park S, Hanna ML, Taylor RTand Droeget MW (1991a) Batch cultivation of Methylosinus trichosporium OB3b. I: production of soluble methane monooxygenase. Biotechnol Bioeng 38:423–433
Park S, Hanna ML, Taylor RT, Droege ML (1991b) Batch cultivation of Methylosinus trichosporium OB3b. II: production of particulate methane monooxygenase. Biotechnol Bioeng 40:151–157
Pilkington SJ, Dalton H (1990) Soluble methane monooxygenase from Methylococcus capsulatus (Bath). Methods Enzymol 188:181–190
Richards AO, Stanley SH, Suzuki M, Dalton H (1994) The biotransformation of propylene to propylene oxide by Methylococcus capsulatus (Bath). 3. Reactivation of inactive whole cells to give a high productivity system. Biocatalysis 8:253–267
Rojas A, Duque E, Schmid A, Hurtado A, Ramos J-L, Segura A (2004) Biotransformation in double-phase systems: physiological responses of Pseudomonas putida DOT-T1E to a double phase made of aliphatic alcohols and biosynthesis of substituted catechols. Appl Environ Microbiol 70:3637–3643
Shah NN, Hanna ML, Taylor RT (1996) Batch cultivation of Methylosinus trichosporium OB3b 5. Characterization of poly-beta-hydroxybutyrate production under methane-dependent growth conditions. Biotechnol Bioeng 49:161–171
Sipkema EM, de Koning W, Ganzeveld KJ Janssen DB, Beenackers AACM (2000) NADH-regulated metabolic model for growth of Methylosinus trichosporium OB3b. Model presentation, parameter estimation, and model validation. Biotechnol Prog 16:176–188
Smith TJ, Dalton H (2004) Biocatalysis by methane monooxygenase and its implications for the petroleum industry. Stud Surf Sci Catal 151:177–192
Smith TJ, Slade SE, Burton NP, Murrell JC, Dalton H (2002) An improved system for protein engineering of the hydroxylase component of soluble methane monooxygenase. Appl Environ Microbiol 68:5265–5273
Sugimori D, Takeguchi M, Okura I (1995) Biocatalytic methanol production from methane with Methylosinus trichosporium Ob3B—an approach to improve methanol accumulation. Biotechnol Lett 17:783–784
Takeguchi M, Okura I (2000) Role of iron and copper in particulate methane monooxygenase of Methylosinus trichosporium OB3b. Catal Surv Japan 4:51–63
Theisen AR, Murrell JC (2005) Facultative methanotrophs revisited. J Bacteriol 187:4303–4305
Witholt B, de Smet MJ, Kingma J, van Beilen JB, Kok M, Lageveen RG, Eggink G (1990) Bioconversion of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Tibtech 8:46–52
Xing X-H, Wu H, Luo MF, Wang BP (2006) Effects of organic chemicals on growth of Methylosinus trichosporium OB3b. Biochem Eng J 31:113–117
Yamashita S, Satoi M, Iwasa Y, Honda K, Sameshima Y, Omasa T, Kato J, Otake H (2007) Utilization of hydrophobic bacterium Rhodococcus opacus B-4 as whole-cell catalyst in anhydrous organic solvents. Appl Microbiol Biotechnol 74:761–767
Yeager CM, Arthur KM, BottomLey PJ, Arp D (2004) Trichloroethylene degradation by toluene-oxidizing bacteria grown on non-aromatic substrates. Biodegradation 15:19–28
Yu SSF, Chen KHC, Tseng MYH, Wang YS, Tseng CF, Chen YJ, Huang DS, Chan SI (2003) Production of high-quality particulate methane monooxygenase in high yields from Methylococcus capsulatus (Bath) with a hollow-fiber membrane bioreactor. J Bacteriol 185:5915–5924
Acknowledgments
We thank Prof. Ichiro Okura of Tokyo Institute of Technology of Japan for donating M. trichosporium OB3b. This work was supported by 863 Plan of Ministry of Science and Technology of China (no. 2006AA02Z203), the National Natural Science foundation of China (no. 2033010), and the PetroChina Foundation (050511-4-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Bing Han and Tao Su contributed equally to this work.
Rights and permissions
About this article
Cite this article
Han, B., Su, T., Wu, H. et al. Paraffin oil as a “methane vector” for rapid and high cell density cultivation of Methylosinus trichosporium OB3b. Appl Microbiol Biotechnol 83, 669–677 (2009). https://doi.org/10.1007/s00253-009-1866-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1866-2