Abstract
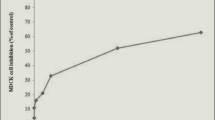
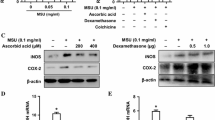
It has been suggested that renal tubular cell damage induced by oxalic acid, one of the components of urinary calculi, may be involved in a variety of ways in the development of urolithiasis. During our study on a calculus related protein, renal prothrombin fragment-1 (RPTF-1), we noted that this is an inflammation related substance that mediates an acute inflammatory reaction, one of the original roles of prothrombin. RPTF-1 is a part of prothrombin that is a coagulation factor known to be expressed in the renal tubule. We examined whether oxalic acid may cause cytotoxic effects on tubular epithelial cells and whether such chemical stimulation may promote the translation of RPTF-1 mRNA into RPTF-1 proteins. We used Madin-Darby canine kidney (MDCK) cells derived from the distal tubule of a dog kidney. In this study, the effects of oxalic acid in culture solution at different concentrations on cytotoxicity were assessed using a MTT assay. The location of active oxygen species was identified using dichlorofluorescein diacetate. After the prothrombin sequence of RPTF-1 was confirmed in MDCK cells, RPTF-1 mRNA expression was determined by RT-PCR. The gene sequence of the same promoter area was ligated, and a luciferase sequence was inserted downstream of the vector. The target sequence was transfected into MDCK cells and the relation between oxalic acid and prothrombin promoter was examined. In addition, the variable expression of RPTF-1 mRNA was quantitatively compared depending on oxalic acid concentrations using real-time PCR. When cytotoxicity was investigated, cells were not damaged but, by contrast, were stimulated and activated under oxalic acid below a certain concentration. The relation between cytotoxicity on the cultured MDCK cell membrane and active oxygen species was confirmed. Luminescence in MDCK cells containing the luciferase gene was detected by the addition of oxalic acid, which activated the prothrombin promoter. A part of the prothrombin gene sequence in the MDCK cells was detected and an increase in the expression of RPTF-1 mRNA in MDCK cells by the addition of oxalic acid was confirmed using real-time PCR. Increased expression of prothrombin by adding oxalic acid has already been demonstrated in previous studies. In this study, however, RPTF-1 mRNA was promoted by oxalic acid and a direct association between oxalic acid and RPTF-1 is indicated. This finding shows that increased oxalic acid in urine induces the expression of RPTF-1 in tubular epithelial cells and thereby causes the generation of active oxygen species.





Similar content being viewed by others
References
Marangella M, Vitale C, Petarulo M et al. (2000) Renal stone: from metabolic to physicochemical abnormalities. How useful are inhibitors? J Nephrol 13 [Suppl 3] : s51
Khan SR, Hackett RL (1985) Calcium oxalate urolithiasis in the rat: is it a model for human stone disease? A review of recent literature. Scanning Electron Microsc 2: 759
Scheid C, Koul H, Hill WA et al. (1996) Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int 49: 413
Hackett RL, Shevock PN, Khan SR (1994) Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res 22: 197
Degen SJ, Davie EW (1987) Human prothrombin (F2) gene, complete cds, and Alu and KpnI repeats. Biochemistry 26: 6165
Hackett RL, Shevock PN, Khan SR (1994) Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res 22: 197
Thamilselvan S, Khan SR (2000) Free radical scavengers, catalase and superoxide dismutase, provide protection from oxalate-associated injury. Urolitiasis 2000, vol 1: 229
Wiessner JH, Hasegawa AT, Hung LY et al. (2001) Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int 59: 637
Scheid C, Honeyman T, Kohjimoto Y et al. (2000) Oxalate-induced changes in renal epithelial cell function: role in stone disease. Mol Urol 4: 371
Suzuki K, Moriyama M, Nakajima C et al. (1994) Isolation and partial characterization of crystal matrix protein as a potent inhibitor of calcium oxalate crystal aggregation: evidence of activation peptide of human prothrombin. Urol Res 22: 45
Nakajima C, Suzuki K, Tsugawa, R (1996): Inhibitory potency of crystal matrix protein on the crystallization of calcium oxalate. Int J Urol 3 [Suppl 1]: S76
Moriyama M, Suzuki K, Tsugawa R (1995) Immunohistochemical study using anti-PIVKA II monoclonal antibody in human kidney tissues. J Kanazawa Med Univ 20: 42
Shiba N, Moriyama M, Suzuki K (1999) Gene expression and polymorphism of prothrombin in the human kidney. J Kanazawa Med Univ 24: 305
Suga K, Moriyama M, Suzuki K (1999) Expression of prothrombin mRNA in normal and stone forming rat kidney. J Kanazawa Med Univ 24: 282
Moriyama MT, Glenton PA, Khan SR (2001) Expression of inter-alpha inhibitor related proteins in kidneys and urine of hyperoxaluric rats. J Urol 165: 1687
Iida S, Peck AB, Johnson-Tardieu J et al. (1999) Temporal changes in mRNA expression for bikunin in the kidneys of rats during calcium oxalate nephrolithiasis. J Am Soc Nephrol 10: 986
Hagar H, Ueda N, Shah SV (1996) Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol 271: F209
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54: 237
Holmgren A (1989) Thioredoxin and glutaredoxin systems. J Biol Chem 264: 13963
Mitsui A, Hirakawa T, Yodoi J (1992) Reactive oxygen-reducing and protein-refolding activities of adult T cell leukemia-derived factor/human thioredoxin. Biochem Biophys Res Commun 186: 1220
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moryama, M.T., Domiki, C., Miyazawa, K. et al. Effects of oxalate exposure on Madin-Darby canine kidney cells in culture: renal prothrombin fragment-1 mRNA expression. Urol Res 33, 470–475 (2005). https://doi.org/10.1007/s00240-005-0510-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-005-0510-6