Abstract
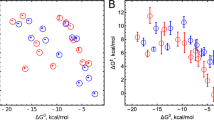
Steady-state and single-turnover kinetics for the oxidation of the N-substituted phenothiazines (PTs) and phenoxazines (POs) catalyzed by fungal Coprinus cinereus peroxidase and Polyporus pinsitus laccase were investigated at pH 4–10. In the case of peroxidase, an apparent bimolecular rate constant (expressed as k cat/K m) varied from 1×107M−1s−1to 2.6×108M−1s−1 at pH 7.0. The constants for PO oxidation were higher in comparison to PT. pH dependence revealed two or three ionizable groups with pK a values of 4.9–5.7 and 7.7–9.7 that significantly affected the activity of peroxidase. Single-turnover experiments showed that the limiting step of PT oxidation was reduction of compound II and second-order rate constants were obtained which were consistent with the constants at steady-state conditions. Laccase-catalyzed PT and PO oxidation rates were lower; apparent bimolecular rate constants varied from 1.8×105M−1s−1 to 2.0×107M−1s−1 at pH 5.3. PO constants were higher in comparison to PT, as was the case with peroxidase. The dependence of the apparent bimolecular constants of compound II or copper type 1 reduction, in the case of peroxidase or laccase, respectively, was analyzed in the framework of the Marcus outer-sphere electron-transfer theory. Peroxidase-catalyzed reactions with PT, as well as PO, fitted the same hyperbolic dependence with a maximal oxidation rate of 1.6×108M−1s−1 and a reorganization energy of 0.30 eV. The respective parameters for laccase were 5.0×108M−1s−1 and 0.29 eV.
Similar content being viewed by others
References
Dunford HB, Adeniran AJ (1986) Arch Biochem Biophys 251: 536–542
Kersten PJ, Kalyanaraman B, Hammel KE, Reinhammar B, Kirk TK (1990) Biochem J 268: 475–480
Kelder PP, de Mol NJ, Fischer MJE, Janssen LHM (1994) Biochim Biophys Acta 1205: 230–238
Folkes LK, Candeias LP (1997) FEBS Lett 412: 305–308
Candeias LP, Folkes LK, Wardman P (1997) Biochemistry 36: 102–108
Krikstopaitis K, Kulys J, Pedersen AH, Schneider P (1998) Acta Chem Scand 52: 469–474
Trudeau F, Daigle F, Leech D (1997) Anal Chem 69: 882–886
Li K, Xu F, Eriksson K-EL (1999) Appl Environ Microbiol 65: 2654–2660
Schneider P, Pedersen AH (1995) Int Pat Appl WO 95/01426; (1995) Chem Abstr
Schneider P, Ebdrup S (1994) Int Pat Appl WO 94/12621; (1994) Chem Abstr
Xu F (1996) Biochemistry 35: 7608–7614
Sakurada J, Sekiguchi R, Sato K, Hosoya T (1990) Biochemistry 29: 4093–4098
Candeias LP, Folkes LK, Wardman P (1997) Biochemistry 36: 7081–7085
Farhangrazi ZS, Copeland BR, Nakayama T, Amachi T, Yamazaki I, Powers LS (1994) Biochemistry 33: 5647–5652
Kulys J, Razumas V, Kazlauskaite J, Marcinkeviciene J, Buch-Rasmussen T, Hansen HE, Bechgaard K (1994) J Mol Catal 91: 407–420
Goodwin D, Yamazaki Y, Aust SD, Grover TA (1995) Anal Biochem 231: 333–338
Andersen MB, Hsuanyu Y, Welinder KG, Schneider P, Dunford HB (1991) Acta Chem Scand 45: 1080–1086
Abelskov AK, Smith AT, Rasmussen CB, Dunford HB, Welinder KG (1997) Biochemistry 36: 9453–9463
Fukuyama K, Sato K, Itakura H, Takahashi S, Hosoya TJ (1997) J Biol Chem. 272: 5752–5756
Neri F, Kok D, Miller MA, Smulevich G (1997) Biochemistry 36: 8947–8953
Banci L, Carloni P, Savellini GG (1994) Biochemistry 33: 12356–12366
Patel PK, Mondal MS, Modi S, Behere DV (1997) Biochim Biophys Acta 1339: 79–87
Marcus RA, Sutin N (1985) Biochim Biophys Acta 811: 265–322
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) Biochim Biophys Acta 1292: 303–311
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulys, J., Krikstopaitis, K. & Ziemys, A. Kinetics and thermodynamics of peroxidase- and laccase-catalyzed oxidation of N-substituted phenothiazines and phenoxazines. JBIC 5, 333–340 (2000). https://doi.org/10.1007/PL00010662
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/PL00010662