Abstract.
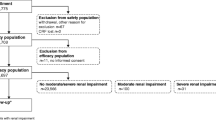
The safety profile of ProHance in special populations was evaluated by analyzing data extracted from the database of phase I-III studies which included data on 2,656 ProHance injections of which 119 in pediatric patients, 814 in elderly patients and 30 in patients with varying degrees of renal impairment (moderate, severe or end stage requiring hemodialysis). ProHance was administered at doses ranging from 0.1 mmol/kg to 0.3 mmol/kg and was found to be safe in all patient populations irrespective of age and of pre-existing renal impairment. There appeared to be no correlation between incidence of adverse events and dose level in these special populations and the higher dose level of 0.3 mmol/kg could be safely administered also to patients with end stage renal disease requiring hemodialysis, from whom the contrast medium was rapidly and efficiently dialysed.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshikawa, K., Davies, A. Safety of ProHance in special populations. Eur Radiol 7 (Suppl 5), S246–S250 (1997). https://doi.org/10.1007/PL00006901
Issue Date:
DOI: https://doi.org/10.1007/PL00006901