Summary
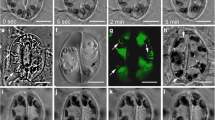
We studied the mechanism controlling the organization of actin filaments (AFs) inHydrocharis root hair cells, in which reverse fountain streaming occurs. The distribution of AFs and microtubules (MTs) in root hair cells were analyzed by fluorescence microscopy and electron microscopy. AFs and MTs were found running in the longitudinal direction of the cell at the cortical region. AFs were observed in the transvacuolar strand, but not MTs. Ultrastructural studies revealed that AFs and MTs were colocalized and that MTs were closer to the plasma membrane than AFs. To examine if MTs regulate the organization of AFs, we carried out a double inhibitor experiment using cytochalasin B (CB) and propyzamide, which are inhibitors of AFs and MTs, respectively. CB reversibly inhibited cytoplasmic streaming while propyzamide alone had no effect on it. However, after treatment with both CB and propyzamide, removal of CB alone did not lead to recovery of cytoplasmic streaming. In these cells, AFs showed a meshwork structure. When propyzamide was also removed, cytoplasmic streaming and the original organization of AFs were recovered. These results strongly suggest that MTs are responsible for the organization of AFs inHydrocharis root hair cells.
Similar content being viewed by others
Abbreviations
- AF:
-
actin filament
- MT:
-
microtubule
- CB:
-
cytochalasin B
- S-1:
-
myosin subfragment-1
- PMSF:
-
phenylmethanesulfonyl fluoride
- EGTA:
-
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- PIPES:
-
piperazine-N,N′-bis(2-ethanesulfonic acid)
References
Abdella PM, Smith PK, Royer GP (1979) A new cleavable reagent for cross linking and reversible immobilization of proteins. Biochem Biophys Res Commun 87: 734–742
Akashi T, Izumi K, Nagano E, Enomoto M, Mizuno K, Shibaoka H (1988) Effects of propyzamide on tobacco cell microtubules in vivo and in vitro. Plant Cell Physiol 29: 1053–1062
Amstel ANMV, Derksen J (1993) The complex helical texture of secondary cell wall ofUrtica dioica root hairs is not controlled by microtubules: a quantitative analysis. Acta Bot Neerl 42: 141–151
Collings DA, Wasteneys GO, Williamson RE (1996) Actin-microtubule interactions in the algaNitella: analysis of the mechanism by which microtubule depolymerization potentiates cytochalasin’s effects on streaming. Protoplasma 191: 178–190
Emons AMC (1987) The cytoskeleton and secretory vesicles in root hairs ofEquisetum andLimnobium and cytoplasmic streaming in root hairs ofEquisetum. Ann Bot 60: 625–632
—, Wolters-Arts AMC, Traas JA, Derksen J (1990) The effect of colchicine on microtubules and microfibrils in root hairs. Acta Bot Neerl 39: 19–27
Ferhat L, Represa A, Bernard A, Ben-Ari Y, Khrestchatisky M (1996) MAP2d promotes bundling and stabilization of both microtubules and microfilaments. J Cell Sci 109: 1095–1103
Ishigami M, Nagai R (1980) Motile apparatus inVallisneria leaf cells. II. Effect of cytochalasin B and lead acetate on the rate and direction of streaming. Cell Struct Funct 5: 13–20
Itano N, Hatano S (1991) F-actin binding protein fromPhysarum polycephalum: purification and its capacity for co-bundling of actin filaments and microtubules. Cell Motil Cytoskeleton 19: 244–254
Kakimoto T, Shibaoka H (1987) A new method for preservation of actin filaments in higher plant cells. Plant Cell Physiol 28: 1581–1585
Kamiya N (1959) Protoplasmic streaming. Springer, Wien [Heilbrunn LV, Weber F (eds) Protoplasmatologia, vol VIII, part 3 a]
— (1981) Physical and chemical basis of cytoplasmic streaming. Annu Rev Plant Physiol 32: 205–236
— (1986) Cytoplasmic streaming in giant algal cells: a historical survey of experimental approaches. Bot Mag Tokyo 99: 441–467
Katsuta J, Hashiguchi Y, Shibaoka H (1990) The role of the cytoskeleton in positioning of the nucleus in premitotic tobacco BY-2 cells. J Cell Sci 95: 413–422
Kersey YM, Hepler PK, Palevitz BA, Wessells NK (1976) Polarity of actin filaments in Characean algae. Proc Natl Acad Sci USA 73: 165–167
Kobayashi H, Fukuda H, Shibaoka H (1988) Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in culturedZinnia cells. Protoplasma 143: 29–37
Kohno T, Shimmen T (1988) Accelerated sliding of pollen tube organelles along Characeae actin bundles regulated by Ca2+. J Cell Biol 106: 1539–1543
—, Chaen S, Shimmen T, Chaen S, Shimmen T (1990) Characterization of the translocator associated with pollen tube organelles. Protoplasma 154: 179–183
Lloyd CW (1983) Helical microtubular arrays in onion root hairs. Nature 305: 311–313
— (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu Rev Plant Physiol 38: 119–139
—, Pearce KJ, Rawlins DJ, Ridge RW, Shaw PJ (1987) Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil Cytoskeleton 8: 27–36
Margossian SS, Lowey S (1982) Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85: 55–71
Masuda Y, Takagi S, Nagai R (1991) Protease-sensitive anchoring of microfilament bundles provides tracks for cytoplasmic streaming inVallisneria. Protoplasma 162: 151–159
Nagai R, Hayama T (1979) Ultrastructure of the endoplasmic factor responsible for cytoplasmic streaming inChara internodal cells. J Cell Sci 36: 121–136
Pedrotti B, Colombo R, Islam K (1994) Microtubule associated protein MAP 1A is an actin-binding and crosslinking protein. Cell Motil Cytoskeleton 29: 110–116
Ridge RW (1995) Micro-vesicles, pyriform vesicles and macro-vesicles associated with the plasma membrane in the root hairs ofVicia hirsuta after freeze-substitution. J Plant Res 108: 363–368
Ryu JH, Takagi S, Nagai R (1995) Stationary organization of the actin cytoskeleton inVallisneria: the role of stable microfilaments at the end walls. J Cell Sci 108: 1531–1539
Sattilaro RF (1986) Interaction of microtubule-associated protein 2 with actin filaments. Biochemistry 25: 2003–2009
Seagull RW, Heath IB (1980) The organization of cortical microtubule arrays in the radish root hair. Protoplasma 103: 205–229
Shimmen T (1988) Characean cells as a tool for studying actomyosin-based motility. Bot Mag Tokyo 101: 533–544
— (1992) Mechanisms of cytoplasmic streaming and amoeboid movement. In: Sugi H (ed) Muscle contraction and cell motility: molecular and cellular aspects. Springer, Berlin Heidelberg New York Tokyo, pp 172–205 (Advances in comparative and environmental physiology, vol 12)
—, Tazawa M (1982) Reconstitution of cytoplasmic streaming in Characeae. Protoplasma 113: 127–131
—, Hamatani M, Saito S, Yokota E, Mimura T, Fusetani N, Karaki H (1995) Roles of actin filaments in cytoplasmic streaming and organization of transvacuolar strands in root hair cells ofHydrocharis. Protoplasma 185: 188–193
Staiger CJ, Schliwa M (1987) Actin localization and function in higher plants. Protoplasma 141: 1–12
Szent-Gyorgyi AG (1953) Meromyosins, the subunits of myosin. Arch Biochem Biophys 42: 305–320
Traas JA, Braat P, Emons AMC, Meekes H, Derksen J (1985) Microtubules in root hairs. J Cell Sci 76: 303–320
Uyeda TQP, Furuya M (1987) ATP-induced relative movement between microfilaments and microtubules inMyxomycete flagellates. Protoplasma 140: 190–192
Williamson RE (1975) Cytoplasmic streaming inChara: a cell model activated by ATP and inhibited by cytochalasin B. J Cell Sci 17: 655–668
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tominaga, M., Morita, K., Sonobe, S. et al. Microtubules regulate the organization of actin filaments at the cortical region in root hair cells ofHydrocharis . Protoplasma 199, 83–92 (1997). https://doi.org/10.1007/BF02539809
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02539809