Abstract
Background
Variegate porphyria (VP) is an inherited disorder of heme biosynthesis that results from a partial deficiency of protoporphyrinogen oxidase (PPOX). Patients with VP may experience acute neurovisceral attacks and cutaneous photosensitivity. To date we have characterized 109 VP patients representing 19 VP families in the Finnish population of 5 million, both biochemically and clinically.
Materials and Methods
Mutations were identified by direct sequencing of the patients’ genomic DNA. The effect of the mutations was determined by sequencing the reverse transcriptase polymerase chain reaction (RT-PCR) product amplified from total RNA extracted from the patients’ lymphoblast cell lines and expressing the mutations in E. coli and COS-1 cells.
Results
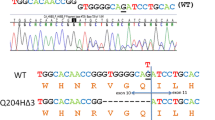
Of the six mutations identified in the PPOX gene, three mutations (IVS2-2a→c, 338G→C, and 470A→C) caused splicing defects, one produced a frameshift (78insC) and two mutations (R152C and L401F) caused amino acid substitutions. In RT-PCR, the IVS2-2a→c mutation caused a retention of a 36-bp fragment in the 3′ end of intron 2, the 338G→ C mutation caused an exon 4 deletion, and the 470A→ C mutation caused an exon 5 deletion with retention of a 19-bp fragment of the 3′ end of intron 5. In both prokaryotic and eukaryotic expression systems, the PPOX activities of five mutants were decreased to 0–5% of the normal activity.
Conclusions
This study describes five novel mutations and one earlier described major mutation among Finnish VP patients. All mutations produced detectable transcripts, but resulted in decreased PPOX activity confirming the causality of the mutations and the biochemical defects in these patients.





Similar content being viewed by others
References
Kappas A, Sassa S, Galbraith RA, Nordmann Y. (1995) The porphyrias. In: Scriver CR, Beaudet A, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited diseases, McGraw-Hill, New York, pp. 2116–2127.
Dailey HA. (1990) Conversion of coproporphyrinogen to proto hem in higher eukaryotes and bacteria: terminal three enzymes. In: Dailey HA, ed. Biosynthesis of heme and chlorophylls, McGraw-Hill, New York, pp. 123–161.
Deybach JC, de Verneuil H, Nordmann Y. (1981) The inherited enzymatic defect in porphyria variegata. Hum. Genet. 58: 425–428.
Mustajoki P. (1980) Variegate porphyria. Twelve years’ experience in Finland. Q. J. Med. 49: 191–203.
Nishimura K, Taketani S, Inokuchi H. (1995) Cloning of a human cDNA for protoporphyrinogen oxidase by complementation in vivo of a hemG mutant of Escherichia coli. J. Biol. Chem. 270: 8076–8080.
Roberts AG, Whatley SD, Daniels J, et al. (1995) Partial characterization and assignment of the gene for protoporphyrinogen oxidase and variegate porphyria to human chromosome 1q23. Hum. Mol. Genet. 4: 2387–2390.
Puy H, Robreau AM, Rosipal R, Nordmann Y, Deybach JC. (1996) Protoporphyrinogen oxidase: complete genomic sequence and polymorphisms in the human gene. Biochem. Biophys. Res. Comm. 226: 226–230.
Meissner PN, Dailey TA, Hift RJ, et al. (1996) A R59W mutation in human protoporphyrinogen oxidase results in decreased enzyme activity and is prevalent in South Africans with variegate porphyria [see comments]. Nat. Genet. 13: 95–97.
Warnich L, Kotze MJ, Groenewald IM, et al. (1996) Identification of three mutations and associated haplotypes in the protoporphyrinogen oxidase gene in South African families with variegate porphyria. Hum. Mol. Genet. 5: 981–984.
Deybach JC, Puy H, Robreau AM, et al. (1996) Mutations in the protoporphyrinogen oxidase gene in patients with variegate porphyria. Hum. Mol. Genet. 5: 407–410.
Lam H, Dragan L, Tsou HC, et al. (1997) Molecular basis of variegate porphyria: a de novo insertion mutation in the protoporphyrinogen oxidase gene. Hum. Genet. 99: 126–129.
Frank J, Christiano A. (1997) Genetic research strategies: a review of the acute porphyrias. Retinoids 13: 88–92.
Frank J, Jugert FK, Kalka K, Goerz G, Merk HF, Christiano AM. (1998) Variegate porphyria: identification of a nonsense mutation in the protoporphyrinogen oxidase gene. J. Invest. Dermatol. 110: 449–451.
Frank J, McGrath J, Lam H, Graham RM, Hawk JL, Christiano AM. (1998) Homozygous variegate porphyria: identification of mutations on both alleles of the protoporphyrinogen oxidase gene in a severely affected proband. J. Invest. Dermatol. 110: 452–455.
Frank J, Lam H, Zaider E, Poh-Fitzpatrick M, Christiano AM. (1998) Molecular basis of variegate porphyria: a missense mutation in the protoporphyrinogen oxidase gene. J. Med. Genet. 35: 244–247.
Roberts AG, Puy H, Dailey TA, et al. (1998) Molecular characterization of homozygous variegate porphyria. Hum. Mol. Genet. 7: 1921–1925.
Frank J, Poh-Fitzpatrick MB, King LE Jr, Christiano AM. (1998) The genetic basis of “Scarsdale Gourmet Diet” variegate porphyria: a missense mutation in the protoporphyrinogen oxidase gene. Arch. Dermatol. Res. 290: 441–445.
Corrigall AV, Hift RJ, Hancock V, et al. (1998) Identification and characterisation of a deletion (537delAT) in the protoporphyrinogen oxidase gene in a South African variegate porphyria family. Hum. Mutat. 12: 403–407.
Whatley SD, Puy H, Morgan RR, (1999) Variegate porphyria in Western Europe. Identification of PPOX gene mutations in 104 families, extent of allelic heterogenity, and absence of correlation between phenotype and type of mutation. Am. J. Hum. Genet. 65: 984–994.
Kauppinen R, Mustajoki P. (1992) Prognosis of acute porphyria: occurrence of acute attacks, precipitating factors, and associated diseases. Medicine 71: 1–13.
Timonen K, Niemi KM, Mustajoki P, Tenhunen R. (1990) Skin changes in variegate porphyria. Clinical, histopathological, and ultrastructural study. Arch. Dermatol. Res. 282: 108–114.
Li F, Lim CK, Peters TJ. (1986) Analysis of urine and faecal porphyrins by HPLC coupled to an advanced automated sample processor. Biomed. Chromatogr. 1: 93–94.
Poh-Fitzpatrick MB. (1980) A plasma porphyrin fluorescence marker for variegate porphyria. Arch. Dermatol. 116: 543–547.
Higuchi R (1989) Simple and rapid preparation of samples for PCR. In: Ehrlich HA, ed. PCR technology. Principles and applications for DNA amplification. New York, Stockton Press, pp. 31–38.
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5294–5299.
Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 9.16–9.19.
Mullis KB, Faloona F. (1987) Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 155: 335–350.
Sanger F, Nicklen S, Coulson AR. (1977) DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74: 5463–5467.
Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. (1989) Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264: 8222–8229.
Krawczak M, Reiss J, Cooper DN. (1992) The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum. Genet. 90: 41–54.
Niwa M, MacDonald CC, Berget SM. (1992) Are vertebrate exons scanned during splice-site selection? Nature 360: 277–280.
Nakai K, Sakamoto H. (1994) Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene 141: 171–177.
Cooper DN. (1993) Human gene mutations affecting RNA processing and translation. Ann. Med. 25: 11–17.
Dailey HA, Dailey TA. (1997) Characteristics of human protoporphyrinogen oxidase in controls and variegate porphyrias. Cell. Molec. Biol. 43: 67–73.
Dailey TA, Meissner P, Dailey HA. (1994) Expression of a cloned protoporphyrinogen oxidase. J. Biol. Chem. 269: 813–815.
Acknowledgments
We thank medical students Ms. Jamelah Tucker and Ms. Katri Kuusisto for their excellent assistance in sequencing. This study was supported by grants from the Magnus Ehrnrooth Foundation, the Finnish Cultural Foundation, the Research Funds and the Clinical Research Institute of the Helsinki University Central Hospital, the Biomedicum Helsinki Foundation, and the University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mutation R152C partly described earlier in Acta Haematologica (1997) 98(suppl. 1) 1–128 (Abstr.)
Rights and permissions
About this article
Cite this article
von und zu Fraunberg, M., Tenhunen, R. & Kauppinen, R. Expression and Characterization of Six Mutations in the Protoporphyrinogen oxidase gene among Finnish Variegate Porphyria Patients. Mol Med 7, 320–328 (2001). https://doi.org/10.1007/BF03402215
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402215