Abstract
Background
Atherosclerosis, the major cause of mortality and invalidity in industrialized countries, is a multi-factorial disease associated with high plasma cholesterol levels and inflammation in the vessel wall. Many different genes have previously been demonstrated in atherosclerosis, although limited numbers of genes are dealt with in each study. In general, data on dynamic gene expression during disease progress is limited and large-scale evaluation of gene expression patterns during atherogenesis could lead to a better understanding of the key events in the pathogenesis of atherosclerosis. We have therefore applied a mouse gene filter array to analyze gene expression in atherosclerotic ApoE-deficient mice.
Materials and Methods
ApoE-deficient mice were fed atherogenic western diet for 10 or 20 weeks and aortas isolated. C57BL/6 mice on normal chow were used as controls. The mRNAs of 15 animals were pooled and hybridized onto commercially available Clontech mouse gene array filters.
Results
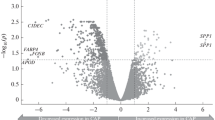
The overall gene expression in the ApoE-deficient and control mice correlated well at both time points. Gene expression profiling showed varying patterns including genes up-regulated at 10 or 20 weeks only. At 20 weeks of diet, an increasing number of up-regulated genes were found in ApoE-deficient mice.
Conclusions
The gene expression in atherogenesis is not a linear process with a maximal expression at advanced lesion stage. Instead, several genes demonstrate a dynamic expression pattern with peaks at the intermediate lesions stage. Thus, detailed evaluation of gene expression at several time points should help understanding the development of atherosclerosis and establishment of preventive intervention.





Similar content being viewed by others
References
Braunwald E. (1997) Shattuck lecture-cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 337: 1360–1369.
Ross R. (1999) Atherosclerosis-an inflammatory disease. N. Engl. J. Med. 340: 115–126.
Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. (1998) The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102: 145–152.
Cybulsky MI, Gimbrone MJ. (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251: 788–791.
Hansson GK, Seifert PS, Olsson G, Bondjers G. (1991) Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler. Thromb. 11: 745–750.
Gerrity RG. (1981) The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am. J. Pathol. 103: 181–190.
Hurt E, Camejo G. (1987) Effect of arterial proteoglycans on the interaction of LDL with human monocyte-derived macrophages. Atherosclerosis 67: 115–126.
Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U.S.A. 92: 3893–3897.
Hansson GK, Holm J, Jonasson L. (1989) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am. J. Pathol. 135: 169–175.
Newby AC, Zaltsman AB. (1999) Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc. Res. 41: 345–360.
Lichtman AH, Cybulsky M, Luscinskas FW. (1996) Immunology of atherosclerosis: the promise of mouse models. Am. J. Pathol. 149: 351–357.
Breslow JL. (1996) Mouse models of atherosclerosis. Science 272: 685–688.
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. (1994) ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14: 133–140.
Hiltunen MO, Niemi M, Yla-Herttuala S. (1999) Functional genomics and DNA array techniques in atherosclerosis research. Curr. Opin. Lipidol. 10: 515–519.
Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. (1992) Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 89: 4471–4475.
Overbergh L, Valckx D, Waer M, Mathieu C. (1999) Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11: 305–312.
Tamayo P, Slonim D, Mesirov J, et al. (1999) Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc. Natl. Acad. Sci. U.S.A. 96: 2907–2912.
Smid-Koopman E, Blok LJ, Chadha-Ajwani S, Helmerhorst TJ, Brinkmann AO, Huikeshoven FJ. (2000) Gene expression profiles of human endometrial cancer samples using a cDNA-expression array technique: assessment of an analysis method. Br. J. Cancer 83: 246–251.
Detmers PA, Hernandez M, Mudgett J, et al. (2000) Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 165: 3430–3435.
Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. (1998) Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 18: 842–851.
Salomon RN, Underwood R, Doyle MV, Wang A, Libby P. (1992) Increased apolipoprotein E and c-fms gene expression without elevated interleukin 1 or 6 mRNA levels indicates selective activation of macrophage functions in advanced human atheroma. Proc. Natl. Acad. Sci. U.S.A. 89: 2814–2818.
Mach F, Schonbeck U, Libby P. (1998) CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis 137(suppl):S89–S95.
Wilcox JN, Smith KM, Williams LT, Schwartz SM, Gordon D. (1988) Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J. Clin. Invest. 82: 1134–1143.
Murry CE, Bartosek T, Giachelli CM, Alpers CE, Schwartz SM. (1996) Platelet-derived growth factor-A mRNA expression in fetal, normal adult, and atherosclerotic human aortas. Analysis by competitive polymerase chain reaction. Circulation 93: 1095–1106.
McCaffrey TA, Fu C, Du B, et al. (2000) High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J. Clin. Invest. 105: 653–662.
Hurt-Camejo E, Andersen S, Standal R, et al. (1997) Localization of nonpancreatic secretory phospholipase A2 in normal and atherosclerotic arteries. Activity of the isolated enzyme on low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 17: 300–309.
Wang X, Li X, Yue TL, Ohlstein EH. (2000) Expression of monocyte chemotactic protein-3 mRNA in rat vascular smooth muscle cells and in carotid artery after balloon angioplasty. Biochim. Biophys. Acta 1500: 41–48.
Kunkel SL. (1999) Through the looking glass: the diverse in vivo activities of chemokines [comment]. J. Clin. Invest. 104: 1333–1334.
McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. (2000) Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289: 1202–1206.
Horiba M, Kadomatsu K, Nakamura E, et al. (2000) Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Invest. 105: 489–495.
Nemoto K, Fukamachi K, Nemoto F, et al. (1998) Gene expression of neurotrophins and their receptors in cultured rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 245: 284–288.
Aloe L, Skaper SD, Leon A, Levi-Montalcini R. (1994) Nerve growth factor and autoimmune diseases. Autoimmunity 19: 141–150.
Sanico AM, Stanisz AM, Gleeson TD, et al. (2000) Nerve growth factor expression and release in allergic inflammatory disease of the upper airways. Am. J. Respir. Crit. Care Med. 161: 1631–1635.
Braun A, Appel E, Baruch R, et al. (1998) Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 28: 3240–3251.
Liuzzo JP, Petanceska SS, Devi LA. (1999) Neurotrophic factors regulate cathepsin S in macrophages and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Mol. Med. 5:334–343.
Lambiase A, Bracci-Laudiero L, Bonini S, et al. (1997) Human CD41 T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immunol. 100: 408–414.
Leon A, Buriani A, Dal Toso R, et al. (1994) Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 91: 3739–3743.
Nakamura Y, Morishita R, Higaki J, et al. (1995) Expression of local hepatocyte growth factor system in vascular tissues. Biochem. Biophys. Res. Commun. 215: 483–488.
Hiscox S, Jiang WG. (1997) Regulation of endothelial CD44 expression and endothelium-tumour cell interactions by hepatocyte growth factor/scatter factor. Biochem. Biophys. Res. Commun. 233: 1–5.
Adams DH, Harvath L, Bottaro DP, et al. (1994) Hepatocyte growth factor and macrophage inflammatory protein 1 beta: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc. Natl. Acad. Sci. U.S.A. 91: 7144–7148.
Niforas P, Chu MD, Bird P. (1996) A retinoic acid/cAMP-responsive enhancer containing a cAMP responsive element is required for the activation of the mouse thrombomodulin-encoding gene in differentiating F9 cells. Gene 176: 139–147.
Wuttge DM, Rommert A, Eriksson U, et al. Induction of CD36 by all-trans Retinoic Acid. Retinoic Acid Receptor Signaling in the Pathogenesis of Atherosclerosis. FASEB J 2001 March 20 [epub ahead of print]
Acknowledgments
We are greatly in debt to Inger Bodin for technical assistance. This project was supported by grants from the Swedish Medical Research Council (12660), the Swedish Heart-Lung Foundation, the Torsten and Ragnar Söderberg Foundation, the Åke Wiberg Foundation, the Magnus Bergvall Foundation, the Foundation for Old Servants, and the Professor Nanna Svartz Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributed by H. Wigzell.
Rights and permissions
About this article
Cite this article
Wuttge, D.M., Sirsjö, A., Eriksson, P. et al. Gene Expression in Atherosclerotic Lesion of ApoE Deficient Mice. Mol Med 7, 383–392 (2001). https://doi.org/10.1007/BF03402184
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402184