Abstract
Background
In the setting of familial melanoma, the presence of atypical nevi, which are the precursors of melanoma, is associated with a nearly 100% risk of developing primary melanoma by age 70. In patients with sporadic melanoma, it is estimated that 40–60% of melanomas develop in contiguous association with atypical nevi. Currently, the only way to prevent atypical nevi from progressing to melanoma is to monitor and excise them as soon as they exhibit changes in their clinical features. Activation of the transcription factor, Stat3, has been linked to abnormal cell growth and transformation as well as to interferon α (IFN-α)-mediated growth suppression in vitro.
Materials and Methods
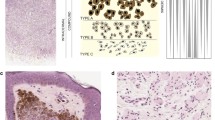
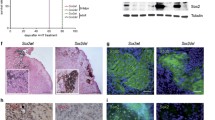
To determine whether IFN-α, used for adjuvant therapy of high-risk, resected melanoma, induces changes in Stat3 in atypical nevi, patients with a clinical history of melanoma who have multiple atypical nevi were treated for 3 months with low-dose IFN-α. Thereupon, the new technology of microscopic spectral imaging and biochemical assays such as electrophoretic mobility shift assays (EMSAs) and immunoblot analysis were used for the study of atypical nevi, obtained before and after IFN-α treatment.
Results
The results of the investigations provided evidence that, as a result of systemic IFN-α treatment, Stat1 and Stat3, which are constitutively activated in melanoma precursor lesions, lose their ability to bind DNA, and as shown in the case of Stat3, become dephosphorylated.
Conclusions
Unlike primary and metastatic melanomas, melanoma precursor lesions cannot be established as cell cultures. Thus, the only way to explore pathways and treatment regimens that might help prevent progression to melanoma is within the context of a melanoma precursor lesion study conducted prospectively. The findings presented here suggest that down-regulation of the transcription factors Stat1 and Stat3 by systemic IFN-α treatment may represent a potential pathway to prevent the activation of gene(s) whose expression may be required for atypical nevus cells to progress to melanoma.



Similar content being viewed by others
References
Clark WH Jr. (1991) Tumor progression and the nature of cancer. Br. J. Cancer 64: 101–112.
Elder DE, Clark WH Jr, Glenitsas R, Guerry D, IV, Halpern AC. (1993) The early and intermediate precursor lesions of tumor progression in the melanocytic system: common acquired nevi and atypical (dysplastic) nevi. Semin. Diagn. Pathol. 10: 18–35.
Greene MH, Clark WH Jr, Tucker MA, et al. (1985) Acquired precursors of cutaneous malignant melanoma. The familial dysplastic nevus syndrome. New Engl. J. Med. 312: 91–96.
Kirkwood JM, Strawderman MH, Ernstoff MS, et al. (1996) Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 14: 7–17.
Grob JJ, Dreno B, de la Salmoniere P, et al. (1998) Randomized trial of interferon alpha-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. Lancet 351: 1905–1910.
Pehamberger H, Soyer HP, Steiner A, et al. (1998) Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. J. Clin. Oncol. 16: 1425–1429.
Darnell JE, Kerr IM, Stark GR. (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421.
Schindler C, Darnell JE Jr. (1995) Transcriptional responses to polypeptide ligands: the Jak-STAT pathway. Annu. Rev. Biochem. 64: 621–651.
Leaman DW, Leung S, Stark GR. (1996). Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 10: 1578–1588.
Ihle JN. (1996) STATs: signal transducers and activators of transcription. Cell 84: 331–334.
Darnell JE. (1997) Stats and gene regulation. Science 277: 1630–1635.
Wang Y, Rao U, Mascari R, et al. (1996) Molecular analysis of melanoma precursor lesions. Cell Growth Differ. 7: 1733–1740.
Wang Y, Becker D. (1997) Antisense targeting of bFGF/FGFR-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat. Med. 3: 887–893.
Levenson RL, Farkas DL. (1997) Digital spectral imaging for histopathology and cytopathology. Proc. SPIE 2983: 123–135.
Farkas DL, Du C, Fisher GW, et al. (1998) Noninvasive image acquisition and advanced processing in optical bioimaging. Comput. Med. Imaging Graph. 22: 89–102.
Sadowski HB, Shuai K, Darnell JE Jr, Gilman MZ. (1993) A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science 261: 1739–17434.
Wong P, Severns CW, Gayer NB, Wright TM. (1994) A unique palindromic element mediates gamma interferon induction of mig gene expression. Mol. Cell. Biol. 14: 914–922.
Tweardy DJ, Wright TM, Ziegler SF, et al. (1995) Granulocyte colony-stimulating factor rapidly activates a distinct STAT-like protein in normal myeloid cells. Blood 86: 4408–4416.
Chakraborty A, White SM, Schaefer TS, et al. (1996) Granulocyte colony-stimulating factor activation of Stat3α and Stat3β in immature normal and leukemic human myeloid cells. Blood 88: 2442–2448.
Farkas DL, Ballou BT, Fisher GW, Taylor DL. (1995) From in vitro to in vivo by dynamic multi-wavelength imaging. Proc. SPIE 2386: 138–149.
Wagner BJ, Hayes TE, Hoban CJ, Cochran BH. (1990) The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 9: 4477–4484.
Hou J, Schindler U, Henzel WJ, et al. (1994) An interleukin-4-induced transcription factor: IL-4 Stat. Science 265: 1701–1706.
Minami M, et al. (1996) Stat3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc. Natl. Acad. Sci. U.S.A. 93: 3963–3966.
Ihara S, Nakajima K, Fukada T, et al. (1997) Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J. 16: 5345–5352.
Fukada T, Hibi M, Yamanaka Y, et al. (1996) Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5: 449–460.
Migone TS, Lin JX, Cereseto A, Mulloy JC, et al. (1995) Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-1. Science 269: 79–81.
Yu CL, Meyer DJ, Campbell GS, et al. (1995) Enhanced DNA binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269: 81–83.
David M, Grimley PM, Finbloom DS, Larner AC. (1993) A nuclear tyrosine phosphatase down-regulates interferon-induced gene expression. Mol. Cell. Biol. 13: 7515–7521.
Haspel RL, Salditt-Georgieff M, Darnell JE Jr. (1996) The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J. 15: 6262–6268.
Chung CD, Liao J, Liu B, et al. (1997) Specific inhibition of Stat3 signal transduction by PIAS3. Science 278: 1803–1805.
Liu B, Liao J, Rao X, et al. (1998) Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. U.S.A. 95: 10626–10631.
Acknowledgments
This work was supported by grants from the National Institutes of Health (D.B. and D.J.T.), the National Science Foundation (D.L.F.), and the Cancer Treatment Research Foundation (D.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirkwood, J.M., Farkas, D.L., Chakraborty, A. et al. Systemic Interferon-α (IFN-α) Treatment Leads to Stat3 Inactivation in Melanoma Precursor Lesions. Mol Med 5, 11–20 (1999). https://doi.org/10.1007/BF03402135
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402135