Abstract
Background
Currently there is no information on the regulation of expression and physiological role of the anti-apoptotic protein Mcl-1 in cells of the melanocytic lineage. This study investigates the regulation and expression of Mcl-1 in human melanoma cells, which was recently found to be induced by betulinic acid, a compound with anti-melanoma and apoptosis-inducing potential.
Materials and Methods
Mcl-1 phosphorthioate anti-sense oligonucleotides were used to investigate the effect of downregulating the expression of Mcl-1. Regulation of Mcl-1 expression was analyzed with the specific PI3-kinase inhibitors LY294002 and wortmannin and the inhibitor of MAP-kinase activation, PD98059. Western blot analysis was performed with anti ERK1/2, Mcl-1, Bak, Bcl-x and Bax antibodies. Activation status of PI-3 kinase and MAP-kinase pathways was investigated using phospho-Akt and phosphorylation-state independent Akt as well as phospho-MAP kinase, phospho-MEK and phospho-GSK-3α/β antibodies.
Results
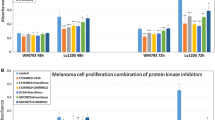
Upregulation of Mcl-1 in human melanoma cells by betulinic acid is mediated via a signal-transduction pathway that is inhibited by LY294002 and wortmannin. Betulinic acid-induced phosphorylation and activation of the Akt protein kinase was inhibited by LY294002. The inhibitor PD98059 reduced expression levels of Mcl-1 in melanoma cells and this effect was counteracted by betulinic acid. Downregulation of Mcl-1 by antisense oligodeoxynucleotides in combination with betulinic treatment led to a synergistic effect regarding growth inhibition.
Conclusions
These results suggest that in human melanoma cells Mcl-1 is (i) of functional relevance for survival and (ii) subject to dual regulation by the MAP-kinase pathway and a pathway involving protein kinase B/Akt, the latter of which is modulated in response to betulinic acid. This study provides an experimental foundation for future therapeutic strategies using anti-Mcl-1 antisense oligonucleotides in human melanoma.







Similar content being viewed by others
References
Selzer E, Pimentel E, Wacheck V, et al. (2000) Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J. Invest. Dermatol. 114: 935–940.
Selzer E, Schlagbauer-Wadl H, Okamoto I, et al. (1998) Expression of Bcl-2 family members in human melanocytes, in melanoma metastases and in melanoma cell lines. Melanoma Res. 8: 197–203.
Pisha E, Chai H, Lee IS, et al. (1995) Discovery of Betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1: 1046–1051.
Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM. (1997) Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur. J. Cancer. 33: 2007–2010.
Rieber M, Strasberg-Rieber M. (1998) Induction of p53 without increase in p21WAF1 in Betulinic acid-mediated cell death is preferential for human metastatic melanoma. DNA. Cell. Biol. 17: 399–406.
Fulda S, Friesen C, Los M, et al. (1997) Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 57: 4956–4964.
Fulda S, Scaffidi C, Susin SA, et al. (1998) Activation of mitochondria and release of mitochondrial apoptogenic factors by Betulinic acid. J. Biol. Chem. 273: 33942–33948.
Fulda S, Jeremias I, Steiner HH, et al. (1999) Betulinic acid: a new cytotoxic agent against malignant brain-tumor cells. Int. J. Cancer. 82: 435–441.
Chao JR, Wang JM, Lee SF, et al. (1998) Mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 18: 4883–4898.
Wang JM, Chao JR, Chen W, et al. (1999) The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol. Cell. Biol. 19: 6195–6206.
Klein JB, Rane MJ, Scherzer JA, et al. (2000) Granulocytemacrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 164: 4286–4291.
Franke TF, Yang SI, Chan TO, et al. (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 2: 727–736.
Hemmings BA. (1997) Akt signaling: linking membrane events to life and death decisions. Science. 275: 628–630.
Marte BM, Downward J. (1997) PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 22: 355–358.
Townsend KJ, Trusty JL, Traupman MA, et al. (1998) Expression of the antiapoptotic MCL1 gene product is regulated by a mitogen activated protein kinase-mediated pathway triggered through microtubule disruption and protein kinase C. Oncogene. 10: 1223–1234.
Stambolic V, Mak TW, Woodgett JR. (1999) Modulation of cellular apoptotic potential: contributions to oncogenesis. Oncogene. 1: 6094–6103.
Huang HM, Huang CJ, Yen JJ. (2000) Mcl-1 is a common target of stem cell factor and interleukin-5 for apoptosis prevention activity via MEK/MAPK and PI-3K/Akt pathways. Blood. 96: 1764–1771.
Kuo ML, Chuang SE, Lin MT, Yang SY. (2001) The involvement of PI 3-K/Akt-dependent up-regulation of Mcl-1 in the prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene. 20: 677–85.
Jansen B, Schlagbauer-Wadl H, Eichler HG, et al. (1997) Activated N-ras contributes to the chemoresistance of human melanoma in severe combined immunodeficiency (SCID) mice by blocking apoptosis. Cancer Res. 57: 362–365.
Toker A, Cantley LC. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 387: 673–676.
Van Weeren PC, de Bruyn KM, de Vries-Smits AM, et al. (1998) Essential role for protein kinase B (PKB) in insulininduced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J. Biol. Chem. 273: 13150–13156.
Rubinfeld B, Robbins P, El-Gamil M, et al. (1997) Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 275: 1790–1792.
Takeda K, Takemoto C, Kobayashi I, et al. (2000) Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum Mol Genet. 1: 125–132.
Kozopas KM, Yang T, Buchan HL, et al. (1993) MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA. 90: 3516–3520.
Tang D, Okada H, Ruland J, et al. (2001) Akt is activated in response to an apoptotic signal. J. Biol. Chem. 10: 30461–30466.
Zhan Q, Bieszczad CK, Bae I, et al (1997) Induction of BCL2 family member MCL1 as an early response to DNA damage. Oncogene. 6: 1031–1039.
Jansen B, Wacheck V, Heere-Ress E, et al. (2000) Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 18: 1728–1733.
Acknowledgments
We thank R. Haslinger for technical assistance. The work in B.J.’s laboratory was supported by grants from: Austrian Science Fund (FWF), Austrian National Bank, “Kommission Onkologie”, “Kamillo Eisner Stiftung”, “Hirtl-Buss Stiftung”, “Hans and Blanca Moser Stiftung”, “Anton Dreher Stiftung”, “Hygienefonds”, “Virologiefonds”.
The work in E.S.’s Laboratory was supported by a “Fonds des Wiener Bürgermeisters” (grant no. 1690).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selzer, E., Thallinger, C., Hoeller, C. et al. Betulinic Acid-induced Mcl-1 Expression in Human Melanoma — Mode of Action and Functional Significance. Mol Med 8, 877–884 (2002). https://doi.org/10.1007/BF03402094
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402094