Abstract
Background
The failure to respond to chemotherapy is a major obstacle in the successful treatment of breast cancer. We have previously shown that anti-HER-2 antisense oligonucleotide (AS HER-2 ODN) treatment was able to sensitize breast cancer cells to various chemotherapeutic agents in vitro irrespective of their HER-2 status, indicating that the use of AS HER-2 ODN therapy for breast cancer is not limited to tumors overexpressing the protein. One of the main drawbacks to the use of antisense therapy in the clinical setting is the lack of an efficient, tumor-targeting, systemic delivery method. We have developed a tumor-specific, ligand-targeting, cationic liposome delivery system designed for systemic gene therapy of cancer. In this study we employ this ligand-liposome strategy to enhance the delivery of the AS Her-2 ODN to breast cancer cells, including those that do not overexpress HER-2, in vitro and in vivo.
Materials and Methods
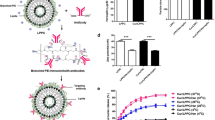
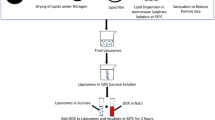
A cationic liposome complex that includes folate as the targeting ligand was designed and optimized for more efficient delivery of AS HER-2 ODN to breast tumors cells in vitro, and more significantly, for systemic delivery with tumor-specific targeting in vivo. Human breast cancer cell line MDA-MB-435, which does not overexpress HER-2, was used to compare the degree of chemosensitization to the taxanes of AS HER-2 ODN delivered via the optimized folateliposome versus commercial Lipofectin. MDA-MB-435 xenograft tumors were also used to evaluate the anti-tumor effect of the combination of systemically delivered folate-liposome-AS HER-2 ODN and docetaxel (Taxotere).
Results
The optimized folate-liposome-AS HER-2 ODN complex significantly increases the response of breast tumor cell lines to conventional chemotherapeutic agents in vitro as compared to AS HER-2 delivered via an unliganded commercially available reagent, Lipofectin. In vivo, the folate-liposome-AS HER-2 ODN complex has prolonged stability in blood and increased uptake in tumors. More significantly, the combination of intravenously administered ligand-liposome-AS HER-2 ODN and docetaxel resulted in a marked inhibition of xenograft growth in an aggressive breast cancer model that does not overexpress HER-2, even after treatment ended.
Conclusions
Although there are other reports of liposomal delivery of AS ODNs, this is the first report of in vivo efficacy against human cancer cells using a tumor-targeting liposome delivery system for systemic AS therapy. Moreover, the increased stability in circulation and anti-tumor efficacy observed were obtained without the need for continuous intravenous infusion. HER-2 is an integral component within a network of cell growth pathways that can affect many different types of tumors where HER-2 may be a contributing factor, such as ovarian, esophageal, and GI malignancies including colon and pancreatic cancers. Therefore, the effectiveness of this therapy with xenograft tumors that do not overexpress HER-2 has the potential to expand the clinical usefulness of this efficacious form of therapy.






Similar content being viewed by others
References
Klapper LN, Kirschbaum MH, Sela M, Yarden Y. (2000) Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv. Cancer Res. 77: 25–79.
Stancovski I, Sela M, Yarden Y. (1994) Molecular and clinical aspects of the Neu/ErbB- 2 receptor tyrosine kinase. Cancer-Treat. Res. 71: 161–191.
Slamon DJ, Godolphin W, Jones LA, et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. (1987) Human breast cancer: correlation of relapse and survival with amplification of the Her-2/neu oncogene. Science 235: 177–182.
Pegram MD, Finn RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ. (1997) The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene 15: 537–547.
Pegram M, Hsu S, Lewis G, et al. (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18: 2241–2251.
Burris HA III. (2001) Docetaxel (Taxotere) plus trastuzumab (Herceptin) in breast cancer. Semin. Oncol. 28: 38–44.
Ring CJ, Blouin P, Martin LA, Hurst HC, Lemoine NR. (1997) Use of transcriptional regulatory elements of the MUC1 and ERBB2 genes to drive tumour-selective expression of a prodrug activating enzyme. Gene Ther. 4: 1045–1052.
Ueno NT, Yu D, Hung MC. (2001) E1A: tumor suppressor or oncogene? Preclinical and clinical investigations of E1A gene therapy. Breast Cancer 8: 285–293.
Rait AS, Pirollo KF, Rait V, Krygier JE, Xiang L, Chang EH. (2001) Inhibitory effects of the combination of HER-2 antisense oligonucleotide and chemotherapeutic agents used for the treatment of human breast cancer. Cancer Gene Ther. 8: 728–739.
Xu L, Pirollo KF, Rait A, Murray A, Chang EH. (1999) Systemic p53 gene therapy in combination with radiation results in human tumor regression. Tumor Targeting 4: 92–104.
Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. (1999) Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Human Gene Ther. 10: 2941–2952.
Xu L, Pirollo KF, Chang EH. (1997) Transferrin-liposome-me-diated p53 sensitization of squamous cell carcinoma of the head and neck to radiation in vitro. Human Gene Ther. 8: 467–475.
Xu L, Pirollo KF, Chang EH. (2001) Tumor-targeted p53 gene therapy enhances the efficacy of conventional chemo/radio-therapy. J. Control Release 6: 115–128.
Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY, USA.
Rait A, Krygier JE, Pirollo KF, Chang EH. (1999) Sensitization of breast cancer cells to taxol by antisense HER-2 oligonucleotides. Antisense Nucleic Acid Drug Dev. 9: 403–408.
Crown JP. (2001) The platinum agents: a role in breast cancer treatment? Semin. Oncol. 28: 28–37.
Nabholtz JM, Tonkin K, Smylie M, Au HJ, Lindsay MA, Mackey J. (2000) Chemotherapy of breast cancer: are the taxanes going to change the natural history of breast cancer? Expert Opin. Pharmacother. 1: 187–206.
O’Leary J VMWCMF. (1998) Taxanes in adjuvant and neoad-juvant therapies for breast cancer. Oncology 12: 23–27.
Agrawal S, Zhao Q. (1998) Mixed backbone oligonucleotides: improvement in oligonucleotide-induced toxicity in vivo. Antisense Nucleic Acid Drug Dev. 8: 135–139.
Glover J, Leeds JM, Mant T, et al. (1997) Phase I safety and pharmacokinetic profile of an intercellular adhesion molecule-1 antisense oligodeoxynucleotide (ISIS 2302). J. Pharmacol. Exp. Ther. 282: 1173–1180.
Rait A, Uhlmann E, Peyman A, Will DW, Chang EH. (2000) Inhibition of Ras p21 synthesis by antisense undecamers with uniform and specifically arranged phosphorothioate linkages. Anticancer Drugs 11: 181–191.
Zamecnik PC, Stephenson ML. (1978) Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. U.S.A. 75: 280–284.
Stephenson ML, Zamecnik PC. (1978) Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. U.S.A. 75: 285–288.
Crooke ST. (1998) Vitravene-another piece in the mosaic. Antisense Nucleic Acid Drug Dev. 8: vii–viii.
Akhtar S, Hughes MD, Khan A, et al. (2000) The delivery of antisense therapeutics. Adv. Drug Deliv. Rev. 44: 3–21.
Tamm I, Dorken B, Hartmann G. (2001) Antisense therapy in oncology: new hope for an old idea? Lancet 358: 489–497.
Akhtar S. (1998) Antisense technology: selection and delivery of optimally acting antisense oligonucleotides. J. Drug Target. 5: 225–234.
Juliano RL, Alahari S, Yoo H, Kole R, Cho M. (1999) Antisense pharmacodynamics: critical issues in the transport and delivery of antisense oligonucleotides. Pharm. Res. 16: 494–502.
Yuen AR, Halsey J, Fisher GA, et al. (1999) Phase I study of an antisense oligonucleotide to protein kinase C-alpha (ISIS 3521/CGP 64128A) in patients with cancer. Clin. Cancer Res. 5: 3357–3363.
Advani R, Fisher G, Lum B, et al. (2000) Coagulation and complement effects of an antisense phosphorothioate oligonucleotide targeting protein kinase C-alpha (ISIS 3521) are schedule and dose dependent. Proc. Am. Soc. Clin. Oncol. 17: 3586–3595.
Nemunaitis J, Holmlund JT, Kraynak M, et al. (1999) Phase I evaluation of ISIS 3521, an antisense oligodeoxynucleotide to protein kinase C-alpha, in patients with advanced cancer. J. Clin. Oncol. 17: 3586–3595.
Flaherty KT, Stevenson JP, O’Dwyer PJ. (2001) Antisense therapeutics: lessons from early clinical trials. Curr. Opin. Oncol. 13: 499–505.
Huang L, Viroonchatapan E. (1999) Introduction. In Non-viral Vectors for Gene Therapy. 3–22, Academic Press, San Diego, CA.
The Journal of Gene Medicine Clinical Trials Database. Available from: URL http://www.wiley.co.uk/wileychi/genmed/clinical. Accessed September 2001.
NCI Clinical Trials Web Site. Available from: URL http://www.clinicaltrials.gov.
Lian T, Ho RJ. (2001) Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 90: 667–680.
Hughes J, Astriab A, Yoo H, et al. (2000) In vitro transport and delivery of antisense oligonucleotides. Meth. Enzymol. 313: 342–358.
Tari AM. (2000) Preparation and application of liposome-incorporated oligodeoxynucleotides. Meth. Enzymol. 313: 372–388.
Kondo Y, Koga S, Komata T, Kondo S. (2000) Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telom-erase RNA component. Oncogene 19: 2205–2211.
Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. (2000) 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 60: 4461–4467.
Endo S, Zeng Q, Burke NA, et al. (2000) TGF-alpha antisense gene therapy inhibits head and neck squamous cell carcinoma growth in vivo. Gene Ther. 7: 1906–1914.
Gokhale PC, Soldatenkov V, Wang FH, et al. (1997) Antisense raf oligodeoxyribonucleotide is protected by liposomal encapsulation and inhibits Raf-1 protein expression in vitro and in vivo: implication for gene therapy of radioresistant cancer. Gene Ther. 4: 1289–1299.
Gokhale PC, McRae D, Monia BP, et al. (1999) Antisense raf oligodeoxyribonucleotide is a radiosensitizer in vivo. Antisense Nucleic Acid Drug Dev. 9: 191–201.
Yoo GH, Hung MC, Lopez-Berestein G, et al. (2001) Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin. Cancer Res. 7: 1237–1245.
Hortobagyi GN, Ueno NT, Xia W, et al. (2001) Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biological effects: a Phase I clinical trial. Clin. Pharmacol. 19: 3422–3433.
Wang S, Lee RJ, Cauchon G, Gorenstein DG, Low PS. (1995) Delivery of antisense oligodeoxyribonucleotides against the human epidermal growth factor receptor into cultured KB cells with liposomes conjugated to folate via polyethylene glycol. Proc. Natl. Acad. Sci. U.S.A. 92: 3318–3322.
Tzahar E, Yarden Y. (1998) The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. [Review] [120 refs]. Biochim. Biophys. Acta 1377: M25–M37.
Acknowledgments
We thank Amy Marshall, Brianna Kalk, and Ekaterina Rait for their assistance in preparation of this manuscript; Ms. Wen-Hua Tang, and the Animal Research Shared Resources Facility for assistance with the animal studies. We also thank the Georgetown University Tissue Culture Core Facility and the Macromolecular Analysis Shared Resources Facility for their assistance. This work was supported in part by the National Foundation for Cancer Research Grant HU0001 (E. H. C.) and SynerGene Therapeutics, Inc. (K. F. P.).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. S. R. and K. F. P. contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Rait, A.S., Pirollo, K.F., Xiang, L. et al. Tumor-targeting, Systemically Delivered Antisense HER-2 Chemosensitizes Human Breast Cancer Xenografts Irrespective of HER-2 Levels. Mol Med 8, 475–486 (2002). https://doi.org/10.1007/BF03402027
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402027