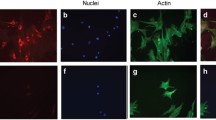
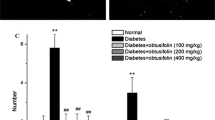
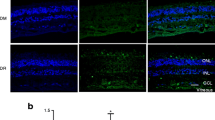
Abstract
Background
Recent observations in the EURODIAB Complications Study demonstrated that markers of insulin resistance are strong risk factors for retinopathy incidence in patients with diabetes. However, the molecular mechanism underlying this remains to be elucidated. In this study, we investigated the influence of palmitate, a major saturated free fatty acid in plasma, on the apoptotic cell death of cultured microvascular endothelial cells (EC) and retinal pericytes.
Materials and Methods
The intracellular formation of reactive oxygen species (ROS) was detected using the fluorescent probe CM-H2DCFDA. DNA synthesis was determined by measuring [3H]-thymidine incorporation into cells. DNA fragmentations of EC were quantitatively analyzed in an enzyme-linked immunosorbent assay, and DNA laddering was evaluated on agarose gel electrophoresis.
Results
Palmitate increased ROS generation in microvascular EC. Furthermore, palmitate significantly inhibited DNA synthesis and induced apoptotic cell death in EC, which were completely prevented by an antioxidant, N-acetylcysteine. Palmitate up-regulated pericyte mRNA levels of a receptor for advanced glycation end products (AGE), and thereby potentiated the apoptotic effects of AGE on pericytes.
Conclusions
The results suggest that palmitate could induce apoptotic cell death in microvascular EC and pericytes through the overgeneration of intracellular ROS, and thus be involved in the development of diabetic retinopathy.






Similar content being viewed by others
References
Cogan DG, Toussaint D, Kuwabara T. (1961) Retinal vascular patterns. IV. Diabetic retinopathy. Arch. Ophthalmol. 66: 366–378.
Yamagishi S, Kobayashi K, Yamamoto H. (1993) Vascular pericytes not only regulate growth, but also preserve prostacyclin-producing ability and protect against lipid peroxide-induced injury of co-cultured endothelial cells. Biochem. Biophys. Res. Commun. 190: 418–425.
Yamagishi S, Hsu CC, Kobayashi K, Yamamoto H. (1993) Endothelin 1 mediates endothelial cell-dependent proliferation of vascular pericytes. Biochem. Biophys. Res. Commun. 191: 840–846.
Podestá F, Romeo G, Liu WH, et al. (2000) Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am. J. Pathol. 156: 1025–1032.
Diabetes Control and Complications Trial Research Group. (1993) The effects of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329: 977–986.
United Kingdom Prospective Diabetes Study Group (UKPDS). (1998) Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet 352: 837–853.
Heart Outcomes Prevention Evaluation (HOPE) Study. (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 355: 253–259.
Orchard TJ, Kuller LH, Forrest KYZ, Becker DJ. (2001) Lipid and blood pressure treatment goals for type 1 diabetes. Diabetes Care 24: 1053–1059.
Shimabukuro M, Zhou YT, Levi M, Unger RH. (1998) Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. U.S.A. 95: 2498–2502.
Bjorntorp P. (1994) Fatty acids, hyperinsulinemia and insulin resistance: which comes first? Curr. Opin. Lipidol. 5: 166–174.
Inoguchi T, Li P, Umeda F, et al. (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945.
Yamagishi S, Hsu CC, Taniguchi M, et al. (1995) Receptor-mediated toxicity to pericytes of advanced glycosylation end products: a possible mechanism of pericyte loss in diabetic microangiopathy. Biochem. Biophys. Res. Commun. 213: 681–687.
Yamagishi S, Amano S, Inagaki Y, et al. (2002) Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 290: 973–978.
Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H. (1998) Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia 41: 1435–1441.
Yamagishi S, Yonekura H, Yamamoto Y, et al. (1999) Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab. Invest. 79: 501–509.
Yamagishi S, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. (2001) Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J. Biol. Chem. 276: 25096–25100.
Yamagishi S, Yamamoto Y, Harada S, Hsu CC, Yamamoto H. (1996) Advanced glycosylation end products stimulate the growth but inhibit the prostacyclin-producing ability of endothelial cells through interactions with their receptors. FEBS Lett. 384: 103–106.
Yamagishi S, Yonekura H, Yamamoto Y, et al. (1997) Advanced glycation end products-driven angiogenesis in vitro. Induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. J. Biol. Chem. 272: 8723–8730.
Yamagishi S, Kawakami T, Fujimori H, et al. (1999) Insulin stimulates the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. Microvasc. Res. 57: 329–339.
Yamagishi S, Fujimori H, Yonekura H, Tanaka N, Yamamoto H. (1999) Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem. Biophys. Res. Commun. 258: 353–357.
Nomura M, Yamagishi S, Harada S, et al. (1995) Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J. Biol. Chem. 270: 28316–28324.
Takeuchi M, Bucala R, Suzuki T, et al. (2000) Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 59: 1094–1105.
Schwartzman RA, Cidlowski JA. (1993) Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocrine Rev. 14: 133–151.
Listenberger LL, Ory DS, Schaffer JE. (2001) Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895.
Chaturvedi N, Songini M, Sjoelie AK, et al. (2001) Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 24: 284–289.
Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. (2000) The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-κB, and by estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 275: 25781–25790.
Yan SD, Schmidt AM, Anderson GM, et al. (1994) Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 269: 9889–9897.
Acknowledgments
This work was supported in part by Grants of Venture Research and Development Centers from the Ministry of Education, Culture, Sports, Science and Technology, Japan; the Suzuken Memorial Foundation, Japan; the Hukuriku University Foundation, Japan; and the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamagishi, Si., Okamoto, T., Amano, S. et al. Palmitate-Induced Apoptosis of Microvascular Endothelial Cells and Pericytes. Mol Med 8, 179–184 (2002). https://doi.org/10.1007/BF03402010
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402010