Abstract
Background
Psychological stress induces rapid and long-lasting changes in blood cell composition, implying the existence of stress-induced factors that modulate hematopoiesis. Here we report the involvement of the stress-associated “readthrough” acetylcholinesterase (AChE-R) variant, and its 26 amino acid C-terminal domain (ARP) in hematopoietic stress responses.
Materials and Methods
We studied the effects of stress, cortisol, antisense oligonucleotides to AChE, and synthetic ARP on peripheral blood cell composition and clonogenic progenitor status in mice under normal and stress conditions, and on purified CD341 cells of human origin. We employed in situ hybridization and immunocytochemical staining to monitor gene expression, and 5-bromo-2-deoxyuridine (BrdU), primary liquid cultures, and clonogenic progenitor assays to correlate AChE-R and ARP with proliferation and differentiation of hematopoietic progenitors.
Results
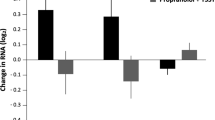
We identified two putative glucocorticoid response elements in the human ACHE gene encoding AChE. In human CD341 hematopoietic progenitor cells, cortisol elevated AChE-R mRNA levels and promoted hematopoietic expansion. In mice, a small peptide crossreacting with anti-ARP antiserum appeared in serum following forced swim stress. Ex vivo, ARP was more effective than cortisol and equally as effective as stem cell factor in promoting expansion and differentiation of early hematopoietic progenitor cells into myeloid and megakaryocyte lineages.
Conclusions
Our findings attribute a role to AChE-R and ARP in hematopoietic homeostasis following stress, and suggest the use of ARP in clinical settings where ex vivo expansion of progenitor cells is required.





Similar content being viewed by others
References
Jern C, Manhem K, Eriksson E, et al. (1991) Hemostatic responses to mental stress during the menstrual cycle. Thromb. Haemost. 66: 614–618.
Sutor AH. (1995) Thrombocytosis in childhood. Semin. Thromb. Hemost. 21: 330–339.
McEwen BS. (1998) Protective and damaging effects of stress mediators. N. Engl. J. Med. 338: 171–179.
Dygai AM, Shakhov VP, Mikhlenko AV, Goldberg ED. (1991) Role of glucocorticoids in the regulation of bone marrow hemopoiesis in stress reaction. Biomed. Pharmacother. 45: 9–14.
Maruyama S, Minagawa M, Shimizu T, et al. (1999) Administration of glucocorticoids markedly increases the numbers of granulocytes and extrathymic T cells in the bone marrow. Cell Immunol. 194: 28–35.
Lansdorp PM. (1995) Telomere length and proliferation potential of hematopoietic stem cells. J. Cell Sci. 108: 1–6.
Burdach S. (1991) The granulocyte/macrophage-colony stimulating factor (GM-CSF): Basic science and clinical application. Klin. Padiatr. 203: 302–310.
Lord KA, Abdollahi A, Hoffman-Liebermann B, Liebermann DA. (1993) Proto-oncogenes of the fos/jun family of transcription factors are positive regulators of myeloid differentiation. Mol. Cell Biol. 13: 841–851.
Dieterlen-Lievre F, Godin I, Pardanaud L. (1997) Where do hematopoietic stem cells come from? Int. Arch. Allergy Immunol. 112: 3–8.
Keller G, Snodgrass R. (1990) Life span of multipotential hematopoietic stem cells in vivo. J. Exp. Med. 171: 1407–1418.
Kaushansky K. (1998) Thrombopoietin and the hematopoietic stem cell. Blood 92: 1–3.
Metcalf D. (1993) The cellular basis for enhancement interactions between stem cell factor and the colony stimulating factors. Stem Cells (Dayt) 11(Suppl 2): 1–11.
Matthews W, Jordan CT, Gavin M, et al. (1991) A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc. Natl. Acad. Sci. U.S.A. 88: 9026–9030.
Small D, Levenstein M, Kim E, et al. (1994) STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD341 human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc. Natl. Acad. Sci. U.S.A. 91: 459–463.
Li CL, Johnson GR. (1994) Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic longterm repopulating cells. Blood 84: 408–414.
Jacobsen SE, Okkenhaug C, Myklebust J, Veiby OP, Lyman SD. (1995) The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: synergistic interactions with inter-leukin (IL) 11, IL-12, and other hematopoietic growth factors. J. Exp. Med. 181: 1357–1363.
McNiece IK, Langley KE, Zsebo KM. (1991) Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp. Hematol. 19: 226–231.
Bernstein ID, Andrews RG, Zsebo KM. (1991) Recombinant human stem cell factor enhances the formation of colonies by CD341 and CD341 lin-cells, and the generation of colony-forming cell progeny from CD341 lin-cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granu-locyte-macrophage colony-stimulating factor. Blood 77: 2316–2321.
Goldberg ED, Dygai AM, Zakharova O, Shakhov VP. (1990) The modulating influence of enkephalins on the bone marrow haemopoiesis in stress. Folia Biol. 36: 319–331.
Karpel R, Ben Aziz-Aloya R, Sternfeld M, et al. (1994) Expression of three alternative acetylcholinesterase messenger RNAs in human tumor cell lines of different tissue origins. Exp. Cell Res. 210: 268–277.
Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM. (1993) Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 41: 31–91.
Lev-Lehman E, Deutsch V, Eldor A, Soreq H. (1997) Immature human megakaryocytes produce nuclear-associated acetylcholinesterase. Blood 89: 3644–3653.
Grisaru D, Lev-Lehman E, Shapira M, et al. (1999) Human osteogenesis involves differentiation-dependent increases in the morphogenically active 3′ alternative splicing variant of acetylcholinesterase. Mol. Cell Biol. 19: 788–795.
Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. (1998) Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell Physiol. 176: 57–66.
Burstein SA, Adamson JW, Harker LA. (1980) Megakaryocy-topoiesis in culture: Modulation by cholinergic mechanisms. J. Cell Physiol. 54: 201–208.
Paoletti F, Mocali A, Vannucchi AM. (1992) Acetyl-cholinesterase in murine erythroleukemia (Friend) cells: Evidence for megakaryocyte-like expression and potential growth-regulatory role of enzyme activity. Blood 79: 2873–2879.
Seidman S, Sternfeld M, Ben Aziz-Aloya R, et al. (1995) Synaptic and epidermal accumulations of human acetyl-cholinesterase are encoded by alternative 3′-terminal exons. Mol. Cell Biol. 15: 2993–3002.
Kaufer D, Friedman A, Seidman S, Soreq H. (1998) Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393: 373–377.
Shohami E, Kaufer D, Chen Y, et al. (2000) Antisense prevention of neuronal damages following head injury in mice. J. Mol. Med. 78: 228–236.
Sternfeld M, Shoham S, Klein O, et al. (2000) Excess “readthrough” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc. Natl. Acad. Sci. U.S.A. 97: 8647–8652.
Grisaru D, Deutsch V, Pick M, et al. (1999) Placing the newborn on the maternal abdomen after delivery increases the volume and CD34 cell content in the umbilical cord blood collected: An old maneuver with new applications. Am. J. Obstet. Gynecol. 180: 1240–1243.
Bertolini F, Battaglia M, Pedrazzoli P, et al. (1997) Megakaryocytic progenitors can be generated ex vivo and safely administered to autologous peripheral blood progenitor cell transplant recipients. Blood 89: 2679–2688.
Pick M, Nagler A, Grisaru D, Eldor A, Deutsch V. (1998) Expansion of megakaryocyte progenitors from human umbilical cord blood using a new two-step separation procedure. Br. J. Haematol. 103: 639–650.
Deutsch VR, Eldor A, Olson T, et al. (1996) Stem cell factor (SCF) synergizes with megakaryocyte colony stimulating activity in post-irradiated aplastic plasma in stimulating human megakaryocytopoiesis. Med. Oncol. 13: 31–42.
Piacibello W, Sanavio F, Garetto L, et al. (1998) Differential growth factor requirement of primitive cord blood hematopoietic stem cell for self-renewal and amplification vs proliferation and differentiation. Leukemia 12: 718–727.
Raina AK, Menn JJ. (1993) Pheromone biosynthesis activating neuropeptide: From discovery to current status. Arch. Insect Biochem. Physiol. 22: 141–151.
Grifman M, Soreq H. (1997) Differentiation intensifies the susceptibility of pheochromocytoma cells to antisense oligodeoxynucleotide-dependent suppression of acetylcholinesterase activity. Antisense Nucleic Acid Drug Dev. 7: 351–359.
Deutsch VR, Olson TA, Nagler A, et al. (1995) The response of cord blood megakaryocyte progenitors to IL-3, IL-6 and aplastic canine serum varies with gestational age. Br. J. Haematol. 89: 8–16.
Kaufer D, Soreq H. (1999) Tracking cholinergic pathways from psychological and chemical stressors to variable neurodeterioration paradigms. Curr. Opin. Neurol. 12: 739–743.
Shapira M, Tur-Kaspa I, Bosgraaf L, et al. (2000) A transcription-activating polymorphism in the ACHE promoter associated with acute sensitivity to anti-acetylcholinesterases. Hum. Mol. Genet. 9: 1273–1281.
Darnell JE Jr, Kerr IM, Stark GR. (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 11415–11421.
McMahon A, Sabban EL. (1992) Regulation of expression of dopamine beta-hydroxylase in PC12 cells by glucocorticoids and cyclic AMP analogues. J. Neurochem. 59: 2040–2047.
Tronche F, Kellendonk C, Kretz O, et al. (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23: 99–103.
Cella N, Groner B, Hynes NE. (1998) Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol. Cell Biol. 18: 1783–1792.
De Vroede M, Beukering R, Spit M, Jansen M. (1998) Rectal hydrocortisone during stress in patients with adrenal insufficiency. Arch. Dis. Child. 78: 544–547.
Blazsek I, Liu XH, Anjo A, et al. (1995) The hematon, a morphogenetic functional complex in mammalian bone marrow, involves erythroblastic islands and granulocytic cobblestones. Exp. Hematol. 23: 309–319.
Ross ME, Evinger MJ, Hyman SE, et al. (1990) Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase promoter using fusion genes introduced into chromaffin cells in primary culture. J. Neurosci. 10: 520–530.
Lopez AJ. (1998) Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu. Rev. Genet. 32: 279–305.
Xie J, McCobb DP. (1998) Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446.
Chan RY, Adatia FA, Krupa AM, Jasmin BJ. (1998) Increased expression of acetylcholinesterase T and R transcripts during hematopoietic differentiation is accompanied by parallel elevations in the levels of their respective molecular forms. J. Biol. Chem. 273: 9727–9733.
Tarasenko LM, Grebennikova VF, Tarasenko VV, et al. (1992) The proteinase and alpha 1-antitrypsin activities in the tissues during emotional stress in rabbits. Fiziol. Zh. 38: 115–117.
Gupta P, Blazar BR, Gupta K, Verfaillie CM. (1998) Human CD341 bone marrow cells regulate stromal production of interleukin-6 and granulocyte colony-stimulating factor and increase the colony-stimulating activity of stroma. Blood 91: 3724–3733.
Jazwiec B, Solanilla A, Grosset C, et al. (1998) Endothelial cell support of hematopoiesis is differentially altered by IL-1 and glucocorticoids. Leukemia 12: 1210–1220.
Sussman JL, Harel M, Silman I. (1993) Three-dimensional structure of acetylcholinesterase and of its complexes with anticholinesterase drugs. Chem. Biol. Interact. 87: 187–197.
Satoh T, Aramini JM, Li S, et al. (1997) Bioactive peptide design based on protein surface epitopes. A cyclic heptapeptide mimics CD4 domain 1 CC ′ loop and inhibits CD4 biological function. J. Biol. Chem. 272: 12175–12180.
Livnah O, Stura EA, Johnson DL, et al. (1996) Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science 273: 464–471.
Johnson DL, Farrell FX, Barbone FP, et al. (1998) Identification of a 13 amino acid peptide mimetic of erythropoietin and description of amino acids critical for the mimetic activity of EMP1. Biochemistry 37: 7699–7710.
Cwirla SE, Balasubramanian P, Duffin DJ, et al. (1997) Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276: 1696–1699.
Conrad PD, Emerson SG. (1998) Ex vivo expansion of hematopoietic cells from umbilical cord blood for clinical transplantation. J. Leukoc. Biol. 64: 147–155.
Galyam N, Grisaru D, Melamed-Book N, Grifman M, Eckstein F, Eldor A, Soreq H. (2001) Complex host cell responses to antisense suppression of ACHE gene expression. Antisense and Nucleic Acid Drug Development in press.
Taniguchi T. (1995) Cytokine signaling through nonreceptor protein tyrosine kinases. Science 268: 251–255.
Jacobson A, Peltz SW. (1996) Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem. 65: 693–739.
Schultz JA, Hoffman WE, Albrecht RF. (1993) Sympathetic stimulation with physostigmine worsens outcome from incomplete brain ischemia in rats. Anesthesiology 79: 114–121.
Harmsen P, Rosengren A, Tsipogianni A, Wilhelmsen L. (1990) Risk factors for stroke in middle-aged men in Goteborg, Sweden. Stroke 21: 223–229.
Inestrosa NC, Alarcon R, Arriagada J, Donoso A, Alvarez J. (1993) Platelets of Alzheimer patients: Increased counts and subnormal uptake and accumulation of [14C]5-hydroxytryptamine. Neurosci. Lett. 163: 8–10.
Snowdon DA, Greiner LH, Mortimer JA, et al. (1997) Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277: 813–817.
Brown LM, Blair A, Gibson R, et al. (1990) Pesticide exposure and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 50: 6585–6591.
Karpel R, Sternfeld M, Ginzberg D, et al. (1996) Overexpression of alternative human acetylcholinesterase forms modulates process extensions in cultured glioma cells. J. Neurochem. 66: 114–123.
Solter D, Gearhart J. (1999) Putting stem cells to work. Science 283: 1468–1470.
Shamblott MJ, Axelman J, Wang S, et al. (1998) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U.S.A. 95: 13726–13731.
Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. (1999) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283: 534–537.
Acknowledgments
The authors are grateful to Dr. Haim Gilon (Jerusalem) for preliminary peptide synthesis, to Drs. David Glick and Shlomo Seidman (Jerusalem) and to Dr. Roger Kornberg (Palo Alto) for reviewing this manuscript, and to Ms. Shoshana Baron for her assistance. Support was by the U.S.-Israel Binational Science Foundation (to H.S.) and the B. Adler Fund, the Israel Ministry of Health (to V.D.). D.G. was the incumbent of a research fellowship from the Tel-Aviv Sourasky Medical Center and of a Meirbaum Award, from Tel-Aviv University.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to this investigation.
Rights and permissions
About this article
Cite this article
Grisaru, D., Deutsch, V., Shapira, M. et al. ARP, A Peptide Derived from the Stress-Associated Acetylcholinesterase Variant, Has Hematopoietic Growth Promoting Activities. Mol Med 7, 93–105 (2001). https://doi.org/10.1007/BF03401943
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401943