Abstract
Background
Apoptosis is a natural process by which damaged and potentially tumorigenic cells are removed. Induction of apoptosis is important in chemotherapy aimed at eliminating cancer cells. We address the mechanisms by which this process can be triggered in cells that are recalcitrant to cell death induced by DNA-damaging agents.
Materials and Methods
Normal human fibroblasts and lymphoblasts, and fibroblasts with defined genetic changes, were treated with DNA-damaging agents and inhibitors of transcription. Western blotting was used to study the expression of some of the key factors involved in the response to DNA damage and the induction of apoptosis, namely, p53, p21WAF1,Cip1, Mdm2, Bax, and CD95 (Fas/APO1). Apoptosis was followed by various criteria, including DNA fragmentation, specific proteolysis, cell morphology, viability, and FACS scan for sub-G1 cells.
Results
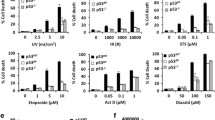
Normal human fibroblasts were more resistant than lymphoblasts to DNA damage-induced apoptosis. The DNA-damaging agents mitomycin C and cisplatin induced rapid apoptosis of fibroblasts with defects in the repair of transcribed DNA, compared with wild-type cells or those with defects in overall genome repair. Short-term treatment with inhibitors of RNA polymerase II transcription, actinomycin D, and α-amanitin induced rapid cell death of normal fibroblasts. These results show that there is a link between defective transcription and apoptosis. Treatments and genetic backgrounds that favored apoptosis were associated with efficient and prolonged induction of p53 and often altered or unbalanced expression of its downstream effectors p21WAF1,Cip1 and Mdm2, whereas there were no changes in Bax or CD95 (Fas/APO1).
Conclusion
Transcription inhibitors increase p53 levels and are better inducers of apoptosis than DNA-damaging agents in some cell types. Apoptosis might be triggered by blocked polymerases and/or faulty expression of downstream effectors.




Similar content being viewed by others
References
Bates S, Vousden KH. (1996) p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 6: 12–18.
Gottlieb TM, Oren M. (1996) p53 in growth control and neoplasia. Biochim. Biophys. Acta 1287: 77–102.
Ko LJ, Prives C. (1996) p53: puzzle and paradigm. Genes Dev. 10: 1054–1072.
Levine AJ. (1997) p53; the cellular gatekeeper for growth and division. Cell 88: 323–331.
Hansen R, Oren M. (1997) p53; from inductive signal to cellular effect. Curr. Opin. Genet. Dev. 7: 46–51.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51: 6304–6311.
Lowe SW, Ruley HE. (1993) Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 7: 535–545.
Lu X, Lane DP. (1993) Differential induction of transcriptionally active p53 following UV or ionizing radiation: Defects in chromosome instability syndromes? Cell 75: 765–778.
Zhan Q, Carrier F, Fornace AJ, Jr. (1993) Induction of cellular p53 activity by DNA-damaging agents and growth arrest [erratum appears in Mol. Cell. Biol. (1993) 13(9): 5928]. Mol. Cell. Biol. 13: 4242–4250.
Demers GW, Foster SA, Haibert CL, Galloway DA. (1994) Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc. Natl. Acad. Sci. U.S.A. 91: 4382–4386.
Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM. (1996) A reversible, p53-de-pendent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 10: 934–947.
Kastan MB, Zhan Q, el-Deiry WS, et al. (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71: 587–597.
Cross SM, Sanchez CA, Morgan CA, et al. (1995) A p53-dependent mouse spindle checkpoint. Science 267: 1353–1356.
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. (1991) Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352: 345–347.
El-Deiry WS, Tokino T, Velculescu VE, et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825.
Del Sal G, Ruaro EM, Utrera R, Cole CN, Levine AJ, Schneider C. (1995) Gas1-induced growth suppression requires a trans-activation-independent p53 function. Mol. Cell. Biol. 15: 7152–7160.
Yuan ZM, Huang Y, Whang Y, et al. (1996) Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature 382: 272–274.
Wen ST, Jackson PK, Van Etten RA. (1996) The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15: 1583–1595.
Symonds H, Krall L, Remington L, et al. (1994) p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78: 703–711.
Lowe SW, Jacks T, Housman DE, Ruley HE. (1994) Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 91: 2026–2030.
Howes KA, Ransom N, Papermaster DS, Lasudry JG, Albert DM, Windle JJ. (1994) Apoptosis or retinoblastoma: Alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53 [erratum appears in Genes Dev. (1994) 8(14): 1738]. Genes Dev. 8: 1300–1310.
Morgenbesser SD, Williams BO, Jacks T, De Pinho RA. (1994) p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens [see comments]. Nature 371: 72–74.
Rowan S, Ludwig RL, Haupt Y, et al. (1996) Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. EMBO J. 15: 827–838.
Sabbatini P, Lin J, Levine AJ, White E. (1995) Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 9: 2184–2192.
Attardi LD, Lowe SW, Brugarolas J, Jacks T. (1996) Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 15: 3693–3701.
Caelles C, Helmberg A, Karin M. (1994) p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes [see comments]. Nature 370: 220–223.
Haupt Y, Rowan S, Shaulian E, Vousden KH, Oren M. (1995) Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 9: 2170–2183.
Wang XW, Vermeulen W, Coursen JD, et al. (1996) The XPB and XPD DNA helicases are components of the p53-mediated apoptosis pathway. Genes Dev. 10: 1219–1232.
Chen X, Ko LJ, Jayaraman L, Prives C. (1996) p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10: 2438–2451.
Wang XW, Yeh H, Schaeffer L, et al. (1995) p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat. Genet. 10: 188–195.
Leveillard T, Andera L, Bissonnette N, et al. (1996) Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 15: 1615–1624.
Miyashita T, Reed JC. (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299.
Owen-Schaub LB, Zhang W, Cusack JC, et al. (1995) Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol. Cell. Biol. 15: 3032–3040.
Muller M, Strand S, Hug H, et al. (1997) Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Invest. 99: 403–413.
Barak Y, Juven T, Haffner R, Oren M. (1993) mdm2 expression is induced by wild-type p53 activity. EMBO J. 12: 461–468.
Perry ME, Piette J, Zawadzki JA, Harvey D, Levine AJ. (1993) The mdm-2 gene is induced in response to UV light in a p53-de-pendent manner. Proc. Natl. Acad. Sci. U.S.A. 90: 11623–11627.
Miyashita T, Krajewski S, Krajewska M, et al. (1994) Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9: 1799–1805.
Zhan Q, Fan S, Bae I, et al. (1994) Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis [erratum appears in Oncogene (1995) 10(6): 1259]. Oncogene 9: 3743–3751.
Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC. (1996) Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene 12: 187–192.
Chen J, Wu X, Lin J, Levine AJ. (1996) mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol. Cell. Biol. 16: 2445–2452.
Haupt Y, Barak Y, Oren M. (1996) Cell type-specific inhibition of p53-mediated apoptosis by mdm2. EMBO J. 15: 1596–1606.
Lowe SW, Ruley HE, Jacks T, Housman DE. (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967.
Yamaizumi M, Sugano T. (1994) UV-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene 9: 2775–2784.
Ljungman M, Zhang F. (1996) Blockage of RNA polymerase as a possible trigger for Uv light-induced apoptosis. Oncogene 13: 823–831.
Vojtesek B, Bartek J, Midgley CA, Lane DP. (1992) An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J. Immunol Methods 151: 237–244.
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 53: 3976–3985.
Roy C, Brown DL, Little JE, et al. (1992) The topoisomerase II inhibitor teniposide (VM-26) induces apoptosis in unstimulated mature murine lymphocytes. Exp. Cell Res. 200: 416–424.
Denizot F, Lang R. (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89: 271–277.
Oberhammer F, Wilson JW, Dive C, et al. (1993) Apoptotic death in epithelial cells: Cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12: 3679–3684.
Graeber TG, Osmanian C, Jacks T, et al. (1996) Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours [see comments]. Nature 379: 88–91.
Allday MJ, Sinclair A, Parker G, Crawford DH, Farrell PJ. (1995) Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 14: 1382–1391.
Allday MJ, Inman GJ, Crawford DH, Farrell PJ. (1995) DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 14: 4994–5005.
Balajee AS, May A, Dianov GL, Friedberg EC, Bohr VA. (1997) Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc. Natl. Acad. Sci. U.S.A. 94: 4306–4311.
Selby CP, Sancar A. (1997) Human transcription-repair coupling factor CSB/ERCC6 is a DNA-stimulated ATPase but is not a helicase and does not disrupt the ternary transcription complex of stalled RNA polymerase II. J. Biol. Chem. 272: 1885–1890.
Friedberg EC. (1996) Cockayne syndrome—a primary defect in DNA repair, transcription, both or neither? Bioessays 18: 731–738.
Venema J, Mullenders LH, Natarajan AT, van Zeeland AA, Mayne LV. (1990) The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc. Natl. Acad. Sci. U.S.A. 87: 4707–4711.
Leadon SA, Cooper PK. (1993) Preferential repair of ionizing radiation-induced damage in the transcribed strand of an active human gene is defective in Cockayne syndrome. Proc. Natl. Acad. Sci. U.S.A. 90: 10499–10503.
Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. (1996) The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 10: 461–477.
Canman CE, Gilmer TM, Coutts SB, Kastan MB. (1995) Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 9: 600–611.
Malcomson RD, Oren M, Wyllie AH, Harrison DJ. (1995) p53-independent death and p53-induced protection against apoptosis in fibroblasts treated with chemotherapeutic drugs. Br. J. Cancer 72: 952–957.
Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. (1996) Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 10: 1945–1952.
Poluha W, Poluha DK, Chang B, et al. (1996) The cyclin-dependent kinase inhibitor p21 (WAF1) is required for survival of differentiating neuroblastoma cells. Mol. Cell. Biol. 16: 1335–1341.
Gorospe M, Cirielli C, Wang X, Seth P, Capogrossi MC, Holbrook NJ. (1997) p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene 14: 929–935.
Walker KK, Levine AJ. (1996) Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc. Natl. Acad. Sci. U.S.A. 93: 15335–15340.
Ruaro EM, Collavin L, Del Sal G, et al. (1997) A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1. Proc. Natl. Acad. Sci. U.S.A. 94: 4675–4680.
Henning KA, Li L, Iyer N, et al. (1995) The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 82: 555–564.
Iyer N, Reagan MS, Wu KJ, Canagarajah B, Friedberg EC. (1996) Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein. Biochemistry 35: 2157–2167.
Yankulov K, Yamashita K, Roy R, Egly JM, Bentley DL. (1995) The transcriptional elongation inhibitor 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole inhibits transcription factor IIH-associated protein kinase. J. Biol. Chem. 270: 23922–23925.
Lu H, Fisher RP, Bailey P, Levine AJ. (1997) The CDK7-cycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Mol. Cell. Biol. 17: 5923–5934.
Milne DM, Campbell LE, Campbell DG, Meek DW. (1995) p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J. Biol. Chem. 270: 5511–5518.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331.
Zanke BW, Boudreau K, Rubie E, et al. (1996) The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 6: 606–613.
Wang Y, Prives C. (1995) Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature 376: 88–91.
Price BD, Hughes-Davies L, Park SJ. (1995) Cdk2 kinase phosphorylates serine 315 of human p53 in vitro. Oncogene 11: 73–80.
Acknowledgments
We are grateful to Jan H. Hoeijmakers, Nicholas J. Jaspers, David P. Lane, and Gilbert De Murcia for generously providing us with cells and antibodies and to Sagar Sengupta for kind help. We thank Thierry Léveillard and Sagar Sengupta for critical reading of the manuscript, members of the Gene Medecine Department of Rhone-Poulenc Rohrer (especially Bruno Tocque, Laurent Bracco, Laurent Debussche, and Emmanuel Conseiller) for continual help and encouragement, the staff of the IGBMC facilities for their invaluable help, and various funding agencies, including: the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Centre Hospitalier Universitaire Régional, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, the Ligue Nationale Française contre le Cancer, the Ligue Régionale (Haut-Rhin) contre le Cancer, the Ligue Régionale (Bas-Rhin) contre le Cancer (the Legs Meyer), and the Bioavenir Program (Ministère de la Recherche et Ministère de l’Industrie).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andera, L., Wasylyk, B. Transcription Abnormalities Potentiate Apoptosis of Normal Human Fibroblasts. Mol Med 3, 852–863 (1997). https://doi.org/10.1007/BF03401721
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401721