Abstract
Background
In Alzheimer’s disease (AD), the main histological lesion is a proteinaceous deposit, the senile plaque, which is mainly composed of a peptide called Aβ. The aggregation process is thought to occur through enhanced concentration of Aβ40 or increased production of the more readily aggregating 42 amino acid-long Aβ42 species.
Materials and Methods
Specificity of the antibodies was assessed by dot blot, Western blot, ELISA, and immunoprecipitation procedures on synthetic and endogenous Aβ produced by secreted HK293 cells. Aβ and p3 production by wild-type and mutated presenilin 1-ex-pressing cells transiently transfected with βAPP751 was monitored after metabolic labeling and immunoprecipitation procedures. Immunohistochemical analysis was performed on brains of sporadic and typical cerebrovascular amyloid angiopathy (CAA) cases.
Results
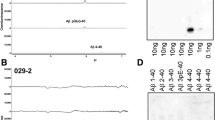
Dot and Western blot analyses indicate that IgG-purified fractions of antisera recognize native and denaturated Aβs. FCA3340 and FCA3542 display full specificity for Aβ40 and Aβ42, respectively. Antibodies immunoprecipitate their respective synthetic Aβ species but also Aβs and their related p3 counterparts endog-enously secreted by transfected human kidney 293 cells. This allowed us to show that mutations on presenilin 1 triggered similar increased ratios of Aβ42 and its p342 counterpart over total Aβ and p3. ELISA assays allow detection of about 25–50 pg/ml of Aβs and remain linear up to 750 to 1500 pg/ml without any cross-reactivity. FCA18 and FCA3542 label diffuse and mature plaques of a sporadic AD case whereas FCA3340 only reveals the mature lesions and particularly labels their central dense core. In a CAA case, FCA18 and FCA3340 reveal leptomeningeal and cortical arterioles whereas FCA3542 only faintly labels such structures.
Conclusions
Polyclonal antibodies exclusively recognizing Aβ40 (FCA3340) or Aβ42 (FCA3542) were obtained. These demonstrated that FAD-linked presenilins similarly affect both p342 and Aβ42, suggesting that these mutations misroute the βAPP to a compartment where γ-secretase, but not α-secretase, cleavages are modified. Overall, these antibodies should prove useful for fundamental and diagnostic approaches, as suggested by their usefulness for biochemical, cell biological, and immunohistochemical techniques.











Similar content being viewed by others
References
Hardy J, Allsop D. (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 12: 383–388.
Glenner GG, Wong CW. (1984) Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120: 885–890.
Masters CL, Simons G, Weinman NA, et al. (1985) Amyloid plaque core protein in Alzheimer’s Disease and Down syndrome. Proc. Natl. Acad. Sci. USA 82: 4245–4249.
Checler F. (1995) Processing of the β-amy-loid precursor protein and its regulation in Alzheimer’s disease. J. Neurochem. 65: 1431–1444.
Selkoe DJ. (1994) Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci. 17: 489–517.
Burdick D, Soreghan B, Kwon M, et al. (1992) Assembly and aggregation properties of synthetic Alzheimer’s A4/β amyloid peptide analogs. J. Biol. Chem. 267: 546–554.
Jarrett JT, Berger EP, Lansbury P, Jr. (1993) The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32: 4693–4697.
Mullan M, Crawford F, Axelman K, et al. (1992) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nature Genet. 1: 345–347.
Citron M, Oltersdorf T, Haass C, et al. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 360: 672–674.
Cai X-D, Golde TE, Younkin SG. (1993) Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science 259: 514–516.
Felsenstein KM, Hunihan LW, Roberts SB. (1994) Altered cleavage and secretion of a recombinant β-APP bearing the Swedish familial Alzheimer’s disease mutation. Nature Genet. 6: 251–256.
Tanzi RE, St. George-Hyslop P, Gusella JF. (1991) Molecular genetics of Alzheimer disease amyloid. J. Biol. Chem. 266: 20579–20582.
Mullan M, Crawford F. (1993) Genetic and molecular advances in Alzheimer’s disease. Trends Neurosci. 16: 398–403.
Suzuki N, Cheung TT, Cai X-D, et al. (1994) An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science 264: 1336–1340.
Levy-Lahad E, Wasco W, Poorkaj P, et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269: 973–977.
Rogaev EI, Sherrington R, Rogaeva EA, et al. (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376: 775–778.
Sherrington R, Rogaev EI, Liang Y, et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375: 754–760.
Borchelt DR, Thinakaran G, Eckman, et al. (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβl-42/1-40 in vitro and in vivo. Neuron 17: 1005–1013.
Citron M, Westaway D, Xia W, et al. (1997) Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nature Med. 3: 67–72.
Duff K, Eckman C, Zehr C, et al. (1996) Increased amyloid-β42(43) in brains expressing mutant presenilin 1. Nature 383: 710–713.
Matsueda GR, Stewart JM. (1981) A p-methylbenzhydrylamine resin for improved solid-phase synthesis of peptides amides. Peptides 2: 45–50.
Cucumel C, Garreau I, Mery J, et al. (1996) Production and characterization of site-directed antibodies against dermorphin and dermorphin-related peptides. Peptides 17: 973–982.
Checler F, Barelli H, Vincent JP. (1989) Tissue distribution of a novel neurotensin-de-grading metallopeptidase. An immunological approach using monospecific polyclonal antibodies. Biochem. J. 257: 549–554.
Schägger H, Von Jagow G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166: 368–379.
Chevallier N, Jiracek J, Vincent B, et al. (1997) Examination of the role of endopeptidase 3.4.24.15 in Aβ secretion by human transfected cells. Br. J. Pharmacol. 121: 556–562.
Marambaud P, Chevallier N, Barelli H, et al. (1997) Proteasome contributes to the α-secre-tase pathway of amyloid precursor protein in human cells. J. Neurochem. 68: 837–845.
Delaere P, He Y, Fayet G, et al. (1993) βA4 deposits are constant in the brain of the oldest old: An immunocytochemical study of 20 French centenarians. Neurobiol. Aging 14: 191–194.
Caporaso L, Gandy SE, Buxbaum JD, et al. (1992) Protein phosphorylation regulates secretion of Alzheimer β/A4 amyloid precursor protein. Proc. Natl. Acad. Sci. USA 89: 3055–3059.
Efthimiopoulos S, Felsenstein KM, Sambamurti K, et al. (1994) Study of the phorbol ester effect on Alzheimer amyloid precursor processing: Sequence requirements and involvement of a cholera toxin sensitive protein. J. Neurosci. Res. 38: 81–90.
Gillespie S, Golde TE, Younkin SG. (1992) Secretory processing of the Alzheimer amyloid β/A4 protein precursor is increased by protein phosphorylation. Biochem. Biophys. Res. Commun. 187: 1285–1290.
Slack BE, Nitsch RM, Livneh E, et al. (1993) Regulation by phorbol esters of amyloid precursor protein release from Swiss 3T3 fibroblasts overexpressing protein kinase Cα. J. Biol. Chem. 268: 21097–21101.
Mori H, Takio K, Ogawara M, et al. (1992) Mass spectrometry of purified amyloid β protein in Alzheimer’s disease. J. Biol. Chem. 267: 17082–17086.
Miller DL, Papayannopoulos LA, Styles J, et al. (1993) Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer’s disease. Arch. Biochem. Biophys. 301: 41–52.
Roher AE, Lowenson JD, Clarke S, et al. (1993) Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and statibility in Alzheimer’s disease. J. Biol. Chem. 268: 3072–3083.
Iwatsubo T, Mann DMA, Odaka A, et al. (1995) Amyloid β protein (Aβ) deposition: Aβ42(43) precedes Aβ40 in Down syndrome. Ann. Neurol. 37: 294–299.
Iwatsubo T, Odaka A, Suzuki N, et al. (1994) Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ mono-clonals: Evidence that an initially deposited species is Aβ42(43). Neuron 13: 45–53.
Cummings BJ, Satou T, Head E, et al. (1996) Diffuse plaques contain C-terminal Aβ42 and not Aβ40: Evidence from cats and dogs. Neurobiol Aging 17: 653–659.
Nakamura S, Tamaoka A, Sawamura N, et al. (1995) Carboxyl end-specific monoclonal antibodies to amyloid β protein (Aβ) subtypes (Aβ40 and Aβ42(43)) differentiate Aβ in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci. Lett. 201: 151–154.
Fukumoto H, Asami-Odaka A, Suzuki N, et al. (1996) Amyloid β protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. Am. J. Pathol. 148: 259–265.
Roher AE, Lowenson JD, Clarke S, et al. (1993) β-amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 90: 10836–10840.
Joachim CL, Duffy LK, Morris JH, et al. (1988) Protein chemical and immunocyto-chemical studies of meningovascular β-amy-loid protein in Alzheimer’s disease and normal aging. Brain Res. 474: 100–111.
Prelli F, Castano E, Glenner GG, et al. Differences between vascular and plaque core amyloid in Alzheimer’s disease. J. Neurochem. 51: 648–651.
Acknowledgments
We wish to acknowledge Drs. Thinakaran and Sisodia (John Hopkins Institute, Baltimore) for providing us with the ΔE9-PS1 cDNA. We sincerely thank Drs. A. Cupo and C. Cucumel for their advice on antigen coupling. We thank J. D. Bardes for animal care and blood sampling, F. Aguila for artwork, and J. Kervella for secretarial assistance. This work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, and by a grant from the Fondation de France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barelli, H., Lebeau, A., Vizzavona, J. et al. Characterization of New Polyclonal Antibodies Specific for 40 and 42 Amino Acid-Long Amyloid β Peptides: Their Use to Examine the Cell Biology of Presenilins and the Immunohistochemistry of Sporadic Alzheimer’s Disease and Cerebral Amyloid Angiopathy Cases. Mol Med 3, 695–707 (1997). https://doi.org/10.1007/BF03401708
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401708