Abstract
Background
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by the deposition of extracellular senile plaques composed of amyloid β-peptide (Aβ). Whereas most cases of AD occur sporadically, about 10% of AD cases are inherited as a fully penetrant autosomal dominant trait. Mutations in the recently cloned Presenilin genes (PS-1 and PS-2) are by far the most common cause of early onset familial AD.
Materials and Methods
Cellular expression of endogenous and overexpressed PS proteins was analyzed by immunocytochemistry and metabolic labeling followed by immunoprecipitation. In vivo phosphorylation sites of PS proteins were analyzed by extensive mutagenesis.
Results
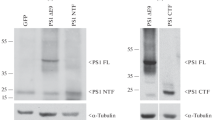
PS-1 as well as PS-2 proteins were localized predominantly within the endoplasmic reticulum (ER). However, small amounts of the PS proteins were detected within the Golgi compartment, where they colocalize with the β-amyloid precursor protein (βAPP). The PS-2 protein was found to be highly phosphorylated. whereas very little phosphorylation was observed for PS-1. The selective phosphorylation of PS-2 occurs exclusively on serine residues. In vivo phosphorylation of PS-2 was mapped to serine residues 7, 9, and 19 within an acidic stretch at the N terminus, which is absent in PS-1. Casein kinase (CK)-l and CK-2 were shown to phosphorylate the N terminus of PS-2 in vitro.
Conclusions
The majority of PS proteins were detected in the ER where little if any proteolytic processing of βAPP was reported. ER retention of PS proteins might occur by intramolecular aggregation. Small amounts of PS proteins were also detected in the Golgi where they colocalized with βAPP. This might suggest that potential interactions between PS proteins and βAPP could occur within the Golgi. Selective phosphorylation of PS-2 proteins within the acidic domain missing in PS-1 indicates differences in the biological functions and regulation of the two highly homologous proteins.









Similar content being viewed by others
References
Selkoe DJ. (1994) Normal and abnormal biology of the β-amyloid precursor protein. Annu. Rev. Neurosci. 17: 489–517.
Cummings BJ, Cotman CW. (1995) Image analysis of β-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet 346: 1524–1528.
Kang J, Lemaire H-G, Unterbeck A, et al. (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736.
Price DL, Sisodia SS, Gandy SE. (1995) Amyloid β amyloidosis in Alzheimer’s disease. Curr. Opin. Neurol. 8: 268–274.
Goate A, Chartier-Harlin MC, Mullan M, et al. (1991) Segregation of a missense mutation in the amyloid precursor gene with familial Alzheimer’s disease. Nature 349: 704–706.
Schellenberg GD, Bird TD, Wijsman E, et al. (1992) Genetic linkage evidence for a familial Alzheimer disease locus on chromosome 14. Science 3: 1–4.
St. George-Hyslop P, Haines J, Rogaev E, et al. (1992) Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nat. Genet. 2: 330–334.
van Broeckhoven C, Backhovens H, Cruts M, et al. (1992) Mapping of a gene predisposing to early-onset Alzheimer’s disease to chromosome 14q24.3. Nat. Genet. 2: 335–339.
Levy-Lahad E, Wijsman EM, Nemens E, et al. (1995) A familial Alzheimer’s disease locus on chromosome I. Science 269: 970–973.
Strittmatter WJ, Roses AD. (1995) Apolipoprotein E and Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 92: 4725–4727.
Mullan M, Crawford F. (1993) Genetic and molecular advances in Alzheimer’s disease. Trends Neurosci. 16: 398–414.
Haass C, Selkoe DJ. (1993) Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 75: 1039–1042.
Citron M, Oltersdorf T, Haass C, et al. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 360: 612–614.
Cai XD, Golde TE, Younkin SG. (1993) Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science 259: 514–516.
Haass C, Hung AY, Selkoe DJ, Teplow DB. (1994) Mutations associated with a locus for familial Alzheimer’s disease result in alternative processing of amyloid β-protein precursor. J. Biol. Chem. 269: 17741–17748.
Suzuki N, Cheung TT, Cai XD, et al. (1994) An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science 264: 1336–1340.
Sherrington R, Rogaev EI, Liang Y, et al. (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375: 754–760.
Levy-Lahad E, Wasco W, Poorkaj P, et al. (1995) Candidate gene for the chromosome I familial Alzheimer’s disease locus. Science 269: 973–977.
Rogaev EI, Sherrington R, Rogaeva EA, et al. (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376: 775–778.
Haass C (1996) Presenile because of presenilin: The presenilin genes and early dementia. Curr. Opin. Neurol. 9: 254–259.
Alzheimer’s Disease Collaborative Group. (1995) The structure of the presenilin 1 (S 182) gene and identification of six novel mutations in early onset AD families. Nat. Genet. 11: 219–222.
Cruts M, Hendriks L, van Broeckhoven C. (1996) The presenilin genes: A new family involved in Alzheimer’s disease pathology. Hum. Mol. Genet. 5: 1449–1455.
Cruts M, Backhovens H, Wang SY, et al. (1995) Molecular genetic analysis of familial early-onset Alzheimer’s disease linked to chromosome 14q24.3. Hum. Mol. Genet. 4: 2363–2371.
Campion D, Flaman JM, Brice A, et al. (1995) Mutations of the presenilin 1 gene in families with early-onset Alzheimer’s disease. Hum. Mol. Genet. 4: 2313–2311.
Wasco W, Pettingell WP, Jondro PD, et al. (1995) Familial Alzheimer’s chromosome 14 mutations. Nat. Med. 1: 848.
Boteva K, Vitek M, Mitsuda H, et al. (1996) Mutation analysis of the presenilin 1 gene in Alzheimer’s disease. Lancet 347: 130–131.
Perez-Tur J, Froelich S, Prihar G, et al. (1995) A mutation in Alzheimer’s disease destroying a splice acceptor site in the presenilin-1 gene. NeuroReport 7: 297–301.
Hutton M, Busfield F, Clark R, et al. (1996) Complete analysis of the presenilin-1 gene in families with early-onset Alzheimer’s disease. NeuroReport 7: 130–131.
Kovacs DM, Fausett HJ, Page KJ, et al. (1996) Alzheimer-associated presenilins 1 and 2: Neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 2: 224–229.
Suzuki T, Nishiyama K, Murayama S, et al. (1996) Regional and cellular presenilin 1 gene expression in human and rat tissues. Biochem. Biophys. Res. Commun. 219: 708–713.
L’Hernault SW, Arduengo PM. (1992) Mutation of a putative sperm membrane protein in Caenorhabditis elegans prevents sperm differentiation but not its associated meiotic divisions. J. Cell Biol. 119: 55–68.
Levitan D, Greenwald I. (1995) Facilitation of lin-12-mediated signaling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 377: 351–354.
Podlisny MB, Tolan DR, Selkoe DJ. (1991) Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer’s disease. Am. J. Pathol. 138: 1423–1435.
Haass C, Hung AY, Selkoe DJ. (1991) Processing of β-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J. Neurosci. 11: 3783–3793.
Hung AY, Haass C, Nitsch RM, et al. (1993) Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J. Biol. Chem. 268: 22959–22962.
Boyle WJ, van der Geer P, Hunter T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201: 110–149.
Haass C, Lemere C, Capell A, et al. (1995) The Swedish mutation causes early onset Alzheimer’s disease by β-secretase cleavage within the secretory pathway. Nat. Med. 1: 1291–1296.
Koo EH, Squazzo S. (1994) Evidence that production and release of amyloid β-protein involves the endocytic pathway. J. Biol. Chem. 269: 17386–17389.
Huttner WB. (1987) Proteine tyrosine sulfation. TIBS 12: 361–363.
Weidemann A, König G, Bunke D, et al. (1989) Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell 57: 115–126.
Roche KW, Tingley WG, Huganir RL. (1994) Glutamate receptor phosphorylation and synaptic plasticity. Curr. Opin. Neurobiol. 4: 383–388.
Buxbaum JD, Gandy SE, Cicchetti P, et al. (1990) Processing of Alzheimer β/A4 amyloid precursor protein: Modulation by agents that regulate protein phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 87: 6003–6006.
Bodenbach L, Fauss J, Robitzki A, et al. (1994) Recombinant human Casein Kinase II: A study with a complete set of subunits (α, α′, and β) site directed autophosphorylation mutants and a bicistronically expressed holoenzyme. Eur. J. Biochem. 220: 262–273.
Bonifacino JS, Cosson P, Sha N, Klausner RD. (1994) Calnexin: a membrane bound chaperone of the endoplasmic reticulum. TIBS 19: 1124–1128.
Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258: 304–307.
Buxbaum JD, Koo EH, Greengard P. (1993) Protein phosphorylation inhibits production of Alzheimer amyloid β/A4 peptide. Proc. Natl. Acad. Sci. U.S.A. 90: 9195–9198.
Lorenz P, Pepperkok R, Ansorge W, Pyerin W. (1993) Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase II subunit β demonstrate participation of the kinase in mitogenic signaling. J. Biol. Chem. 268: 90–97.
Issinger OG. (1993) Casein kinases: Pleiotropic mediators of cellular regulation. Pharmacol. Ther. 59: 1–30.
Jones BG, Thomas L, Molloy SS, et al. (1995) Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14: 5869–5883.
Tartakoff AM, Vassali P. (1983) Lectin-binding sites as markers of Golgi subcompartments: Proximal-to-distal maturation of oligisaccharides. J. Cell Biol. 97: 1243–1248.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB317HA 1737/2-1; MU 467/8-1), by the Boehringer Ingelheim KG. (to CH), by National Institutes of Health Grants AG 06173 and AG 12749 (to DJS), and by the Alzheimer Association of Ontario, the Medical Research Council of Canada and the Canadian Genetic Disease Network (to PSH). We thank Dr. Walter Pyerin for providing human recombinant CK-2, Dr. Edward Koo for providing monoclonal antibodies to βAPP, Dr. Dale Schenk for providing antibody B5, and Dr. Martin Citron for critical comments and discussion.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walter, J., Capell, A., Grünberg, J. et al. The Alzheimer’s Disease-Associated Presenilins Are Differentially Phosphorylated Proteins Located Predominantly within the Endoplasmic Reticulum. Mol Med 2, 673–691 (1996). https://doi.org/10.1007/BF03401652
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401652