Abstract
Background
Advanced glycation end products (AGE), the reactive derivatives of nonenzymatic glucose-protein condensation reactions, are implicated in the multiorgan complications of diabetes and aging. An AGE-specific cellular receptor complex (AGE-R) mediating AGE removal as well as multiple biological responses has been identified. By screening an expression library using antibody against a previously identified component of the AGE-R complex p90, a known partial cDNA clone was isolated with homology to galectin-3, a protein of diverse identity, and member of the galectin family.
Materials and Methods
To explore this unexpected finding, the nature of the interactions between galectin-3 and AGE was studied using intact macrophage-like RAW 264.7 cells, membrane-associated and recombinant galectin-1 through -4, and model AGE-ligands (AGE-BSA, FFI-BSA).
Results
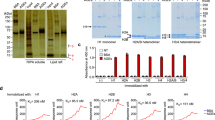
Among the members of this family (galectin-1 through 4), recombinant rat galectin-3 was found to exhibit high-affinity 125I-AGE-BSA binding with saturable kinetics (kD 3.5 × 107 M−1) that was fully blocked by excess unlabeled naturally formed AGE-BSA or synthetic FFI-BSA, but only weakly inhibited by several known galectin-3 ligands, such as lactose. In addition to the p90, immunoprecipitation with anti-galectin-3, followed by 125I-AGE-BSA ligand blot analysis of RAW 264.7 cell extracts, revealed galectin-3 (28 and 32 kD), as well as galectin-3-associated proteins (40 and 50 kD) with AGE-binding activity. Interaction of galectin-3 with AGE-BSA or FFI-BSA resulted in formation of SDS-, and β-mercaptoethanol-insoluble, but hydroxylamine-sensitive high-molecular weight complexes between AGE-ligand, galectin-3, and other membrane components.
Conclusions
The findings point toward a mechanism by which galectin-3 may serve in the assembly of AGE-R components and in the efficient cell surface attachment and endocytosis by macrophages of a heterogenous pool of AGE moieties with diverse affinities, thus contributing to the elimination of these pathogenic substances.









Similar content being viewed by others
References
Bucala R, Vlassara H, Cerami A. (1992) Advanced glycosylation endproducts. Harding JJ, James M, Crabbe C (eds). In: Post-Translational Modifications of Proteins. CRC Press, Boca Raton, FL. Vol. 2, pp. 53–79.
Brownlee M, Vlassara H, Cerami A. (1987) The pathogenetic role of nonenzymatic glycosylation in diabetic complications. Crabbe MJC (ed). In: Diabetic Complications: Scientific and Clinical Aspects. Churchill, Livingstone, London, pp. 94–139.
Dyer DG, Dunn JA, Thorpe SR, et al. (1993) Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest 91: 2463–2469.
Vlassara H, Bucala R, Striker L. (1993) Pathogenic effects of advanced glycosylation; Biochemical, biological, and clinical implications for diabetes and aging. J. Lab. Invest. 70: 138–151.
Brownlee M, Cerami A, Vlassara H. (1988) Advanced glycosylation endproducts in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318: 1315–1321.
Vlassara H, Brownlee M, Cerami A. (1985) High-affinity receptor-mediated uptake and degradation of glucose-modified proteins: A potential mechanism for the removal of senescent macromolecules. Proc. Natl. Acad. Sci. U.S.A. 82: 5588–5592.
Vlassara H, Brownlee M, Cerami A. (1986) Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J. Exp. Med. 164: 1301–1309.
Esposito C, Stern D, Gerlach H, Vlassara H. (1989) Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J. Exp. Med. 170: 1387–1407.
Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H. (1991) Human and rat mesangial cell receptors for glucose-modified proteins: Potential role in kidney tissue remodelling and diabetic nephropathy. J. Exp. Med. 174: 931–939.
Brett J, Schmidt A-M, Zou Y-S, et al. (1993) Tissue distribution of the receptor for advanced glycation endproducts (RAGE): Expression in smooth muscle, cardiac myocytes, and neural tissue in addition to the vasculature. Am. J. Pathol. 143: 1699–1712.
Radoff S, Vlassara H, Cerami A. (1988) Characterization and isolation of a macrophage receptor for proteins modified non-enzymatically by advanced glycosylation endproducts. Arch. Biochem. Biophys. 263: 418–423.
Radoff S, Cerami A, Vlassara H. (1990) Isolation of a surface binding protein specific for advanced glycosylation endproducts from the murine macrophage-derived cell line RAW 264.7. Diabetes 39: 1510–1518.
Yang Z, Makita Z, Horii Y, et al. (1991) Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: Relationship to macrophage receptor for glucose-modified proteins. J. Exp. Med. 174: 515–524.
Imani F, Horii Y, Suthanthiran M, et al. (1993) Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon-γ; Role in tissue remodeling. J. Exp. Med. 178: 2165–2172.
Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ. (1992) Receptor-specific increased mesangial cell extracellular matrix production is mediated by PDGF. Proc. Natl. Acad. Sei. U.S.A. 89: 2873–2877.
Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J. (1992) Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 256:(21): 14987–14997.
Neeper M, Schmidt AM, Brett J, et al. (1992) Cloning and expression of a cell surface receptor for advanced glycosylation endproducts of proteins. J. Biol. Chem. 267: 14998–15004.
Barondes SH, Cooper NW, Gitt MA, Leffler H. (1994) Structure and function of a large family of animal lectins. J. Biol. Chem. 269(33): 20807–20810.
Drickamer K, Taylor ME. (1993) Biology of animal lectins. Annu. Rev. Cell Biol. 9: 237–264.
Barondes SH, Castronovo V, Cooper DNW, et al. (1994) Galectins: A family of animal beta galactoside-binding lectins. Cell 76: 597–598.
Ho MK, Springer TA. (1982) Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 128: 1221–1228.
Jia S, Wang JL. (1988) Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J. Biol. Chem. 263: 6009–6011.
Lindstedt R, Apodaca G, Barondes SH, Mostov K, Leffler H. (1993) Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a non classical secretory pathway. J. Biol. Chem. 268: 11750–11757.
Lotz MM, Andrews Jr CW, Korzelius CA, et al. (1993) Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc. Natl. Acad. Sci. U.S.A. 90: 3466–3470.
Sparrow CP, Leffler H, Barondes SH. (1987) Multiple Soluble β-galactoside-binding lectins from human lung. J. Biol. Chem. 262: 7383–7390.
Frigeri L, Robertson MW, Liu F-T. (1990) Expression of biologically active recombinant rat IgE-binding protein in E. coli. J. Biol. Chem. 265: 20763.
Pongor S, Ulrich PC, Benesath FA, Cerami A. (1984) Aging of proteins: Isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc. Natl. Acad. Sci. U.S.A. 81: 2684.
Palinski W, Koschinsky T, Butler SW, et al. (1995) Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler. Thromb. Vase. Biol. 15(5): 571–582.
Makita Z, Vlassara H, Cerami A, Bucala R. (1992) Immunochemical detection of advanced glycosylation endproducts in vivo. J. Biol. Chem. 267: 5133–5138.
Towbin H, Staehelin T, Gordon J. (1979) Electrophoreirs of proteins from Polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76: 4350–4354.
Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning: Detection and Analysis of Proteins Expressed from Cloned Genes. Cold Spring Harbor Laboratory Press, New York. Vol 3. p. 18.74.
Massa SM, Cooper DNW, Leffler H, Barondes SH. (1993) L-29, an endogenous lectin binds to glycoconjugate ligands with positive cooperativity. Biochemistry 32: 260–267.
Hsu DK, Zuberi R, Liu F-T. (1992) Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 267: 14167.
Farmar JG, Ulrich PC, Cerami A. (1988) Novel pyrroles from sulfite-inhibited Maillard reactions: Insight into the mechanism of inhibition. J. Organic Chem. 53: 2346–9.
Sell DR, Nagaraj RH, Grandhee SK, et al. (1991) Pentosidine—A molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diab. Metab. Rev. 7: 239–251.
Miyata S, Monnier V. (1992) Immunohisto-chemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J. Clin. Invest. 89: 1102–1112.
Knibbs RN, Agrwal N, Wang JL, Goldstein IJ. (1993) Carbohydrate-binding protein 35. J. Biol. Chem. 268(20): 14940–14947.
Cooper DNW, Barondes SH. (1990) Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J. Cell. Biol. 110: 1681–1691.
Vlassara H, Brownlee M, Manogue KR, Dinarello C, Pasagian A. (1988) Cachectin/TNF and IL-1 induced by glucose-modified proteins: Role in normal tissue remodelling. Science 240: 1546–1548.
Vlassara H, Moldawer L, Chan B. (1989) Macrophage/monocyte receptor for non-enzymatically glycosylated proteins is up-regulated by cachectin/tumor necrosis factor. J. Clin. Invest. 84: 1813–1820.
Law SK, Lichtenberg NA, Levine RP. (1979) Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J. Immunol. 123: 1388–1394.
Law SK, Levine RP. (1977) Interaction between the third complement protein and cell surface macromolecules. Proc. Natl. Acad. Sci. U.S.A. 74: 2701–2705.
Pangburn MK. (1992) Spontaneous reformation of the intramolecular thioester in complement protein C3 and low temperature capture of a conformational intermediate capable of reformation. J. Biol. Chem. 267: 8584–8590.
Hatfield PM, Vierstra RD. (1992) Multiple forms of ubiquitin-activating enzyme El from wheat. J. Biol. Chem. 267: 14799–14803.
Woo H-J, Shaw LM, Messier JM, Mercurio AM. (1990) The major non-integrin lamin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J. Biol. Chem. 265: 7097–7099.
Raz A, Pazerini G, Carmi P. (1989) Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 49: 3489–3493.
Acknowledgments
We would like to thank Dr. Kirk Manogue and Dr. Bradley Berger for their help and guidance. We also thank Dr. Hakon Leffler and Dr. Samuel H. Barondes (University of California, San Francisco, CA) for the supply of the galectin 1–4 and their valuable cooperation. Our thanks to Dr. J. L. Wang (Michigan State University) for providing CBP-35, and C-peptide and to Dr. Vincent Monnier (Case Western Reserve, CL) for pyrraline and pentosidine which were also generously provided. These studies were supported in part by The National Institutes of Health Grants AGO-6943 and AGO-9453 to HV.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vlassara, H., Li, Y.M., Imani, F. et al. Identification of Galectin-3 As a High-Affinity Binding Protein for Advanced Glycation End Products (AGE): A New Member of the AGE-Receptor Complex. Mol Med 1, 634–646 (1995). https://doi.org/10.1007/BF03401604
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401604