Abstract
Background
The developmental stage from which stems the malignant B cell population in Burkitt’s lymphoma (BL) is unclear. An approach to answering this question is provided by the sequence analysis of rearranged immunoglobulin (Ig) variable region (V) genes from BL for evidence of somatic mutations, together with a phenotypic characterization. As somatic hypermutation of Ig V region genes occurs in germinal center B cells, somatically mutated Ig genes are found in germinal center B cells and their descendents.
Materials and Methods
Rearranged Vκ region genes from 10 κ-expressing sporadic and endemic BL-derived cell lines (9 IgM and 1 IgG positive) and three κ-expressing endemic BL biopsy specimens were amplified by polymerase chain reaction and sequenced. In addition, VH region gene sequences from these cell lines were determined.
Results
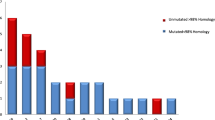
All BL cell lines and the three biopsy specimens carried somatically mutated V region genes. The average mutation frequency of rearranged Vκ genes from eight BL cell lines established from sporadic BL was 1.8%. A higher frequency (6%) was found in five endemic cases (three biopsy specimens and two BL cell lines).
Conclusions
The detection of somatic mutations in the rearranged V region genes suggests that both sporadic and endemic BL represent a B-cell malignancy originating from germinal center B cells or their descendants. Interestingly, the mutation frequency detected in sporadic BL is in a range similar to that characteristic for IgM-expressing B cells in the human peripheral blood and for μ chain-expressing germinal center B cells, whereas the mutation frequency found in endemic BL is significantly higher.


Similar content being viewed by others
References
Magrath I. (1990) The pathogenesis of Burkitt’s lymphoma. Adv. Cancer Res. 55: 133–260.
Klein G, Klein E. (1985) Evolution of tumours and the impact of molecular oncology. Nature 315: 190–195.
Shiramizu B, Barriga F, Neequaye J, et al. (1991) Patterns of chromosomal breakpoint locations in Burkitt’s lymphoma: Relevance to geography and Epstein-Barr virus association. Blood 77: 1516–1526.
Harris NL, Jaffe ES, Stein H, et al. (1994) A revised European-American classification of lymphoid neoplasms: A proposal from the international lymphoma study group. Blood 84: 1361–1392.
Ling NR, Hardie D, Lowe J, Johnson GD, Khan M, MacLennan ICM. (1989) A phenotypic study of cells from Burkitt lymphoma and EBV-B-lymphoblastoid lines and their relationship to cells in normal lymphoid tissues. Int. J. Cancer 43: 112–118.
Gregory CD, Tursz T, Edards CF, et al. (1987) Identification of a subset of normal B cells with a Burkitt’s lymphoma (BL-)-like phenotype. J. Immunol. 139: 313–318.
Kraal G, Weissman IL, Butcher EC. (1982) Germinal centre B cells: Antigen specificity and changes in heavy chain class expression. Nature 298: 377–379.
Feuillard J, Taylor D, Casamayor-Palleja M, Johnson GD, MacLennan ICM. (1995) Isolation and characteristics of tonsil centroblasts with reference to Ig class switching. Intern. Immunol. 7: 121–130.
Snapper CM, Mond JJ. (1993) Toward a comprehensive view of immunoglobulin class switching. Immunol. Today 14: 15–17.
Klein G. (1987) In defense of the “old” Burkitt lymphoma scenario. Adv. Viral Oncol. 7: 207–211.
Stewart AK, Schwartz RS. (1994) Immunoglobulin V regions and the B cell. Blood 83: 1717–1730.
Greaves MF, Hariri G, Newman RA, Sutherland DR, Ritter MA, Ritz J. (1983) Selective expression of the common acute lymphoblastic leukemia (gp100) antigen on immature lymphoid cells and their malignant counterparts. Blood 61: 628–639.
Tedder TF, Clement LT, Cooper MD. (1984) Discontinuous expression of a membrane antigen (HB-7) during B lymphocyte differentiation. Tissue Antigens 24: 140–149.
Schittek B, Rajewsky K. (1992) Natural occurrence and origin of somatically mutated memory B cells in mice. J. Exp. Med. 176: 427–438.
Klein U, Küppers R, Rajewsky K. (1993) Human IgM+IgD + B cells, the major B cell subset in the peripheral blood, express Vκ genes with no or little somatic mutation throughout life. Eur. J. Immunol. 23: 3272–3277.
Klein U, Küppers R, Rajewsky K. (1994) Variable region gene analysis of B cell subsets derived from a 4-year-old child: Somatically mutated memory B cells accumulate in the peripheral blood already at young age. J. Exp. Med. 180: 1383–1393.
Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. (1994) Analysis of somatic mutation in five B cell subsets of human tonsil. J. Exp. Med. 180: 329–339.
Küppers R, Zhao M, Hansmann ML, Rajewsky K. (1993) Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. E.M.B.O. J. 12: 4955–4967.
Mounir S, Guglielmi P, Preud’Homme JL, Nau F, Cogne M. (1990) Alternate splice sites within the human VH gene coding sequences lead to truncated Ig μ-chains. J. Immunol. 144: 342–347.
Klobeck HG, Combriato G, Zachau HG. (1984) Immunoglobulin genes of the κ light chain type from two human lymphoid cell lines are closely related. Nucleic Acids Res. 12: 6995–7006.
Klobeck HG, Meindl A, Combriato G, Solomon A, Zachau HG. (1985) Human immunoglobulin kappa light chain genes of subgroup II and III. Nucleic Acids Res. 13: 6499–6513.
Klobeck HG, Bornkamm GW, Combriato G, Mocikat R, Pohlenz HD, Zachau HG. (1985) Subgroup IV of human immunoglobulin κ light chains is encoded by a single germline gene. Nucleic Acids Res. 13: 6515–6529.
Kato S, Tachibana K, Takayama N, Kataoka H, Yoshida MC, Takano T. (1991) Genetic recombination in a chromosomal t(2;8)(p11; q24) of a Burkitt’s lymphoma cell line, KOBK101. Gene 97: 239–244.
Anderson MLM, Brown L, Kellow JE, Young BD. (1985) Cloning and sequence analysis of an Ig lambda light chain mRNA expressed in the Burkitt’s lymphoma cell line EB4. Nucleic Acids Res. 13: 2931–2941.
Tsujimoto Y, Croce CM. (1984) Molecular cloning of a human immunoglobulin lambda chain variable sequence. Nucleic Acids Res. 12: 8407–8414.
Sun LHK, Croce CM, Showe LC. (1985) Cloning and sequencing of a rearranged Vlambda gene from a Burkitt’s lymphoma cell line expressing kappa light chains. Nucleic Acids Res. 13: 4921–4934.
Carroll WL, Yu M, Link MP, Korsmeyer SL. (1989) Absence of Ig V region gene somatic hypermutation in advanced Burkitt’s lymphoma. J. Immunol. 143: 692–698.
Riboldi P, Gaidano G, Schettino EW, et al. (1994) Two acquired immunodeficiency syndrome-associated Burkitt’s lymphomas produce specific anti-i IgM cold agglutinins using somatically mutated VH4-21 segments. Blood 83: 2952–2961.
Ng VL, Hurt MH, Fein CL, et al. (1994) IgMs produced by two acquired immune deficiency syndrome lymphoma cell lines: Ig binding specificity and VH-gene putative somatic mutation analysis. Blood 83: 1067–1078.
Jain R, Roncella S, Hashimoto S, et al. (1994) A potential role for antigen selection in the clonal evolution of Burkitt’s lymphoma. J. Immunol. 153: 45–52.
Schäble KF, Zachau HG. (1993) The variable genes of the human immunoglobulin k locus. Biol. Chem. Hoppe-Seyler 374: 1001–1022.
Chomczynski P, Sacchi N. (1987) Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156–160.
Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning. A Laboratory Manual. 2nd Ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G. (1992) The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J. Mol. Biol. 227: 776–798.
Rechavi G, Bienz B, Ram D, et al. (1982) Organization and evolution of immunoglobulin VH gene subgroups. Proc. Natl. Acad. Sci. U.S.A. 79: 4405–409.
Sanz I, Kelly P, Williams C, Scholl S, Tucker P, Capra JD. (1989) The smaller human VH gene families display remarkably little polymorphism. E.M.B.O. J. 8: 3741–3748.
Andris JS, Brodeur BR, Capra JD. (1993) Molecular characterization of human antibodies to bacterial antigens: Utilization of the less frequently expressed VH2 and VH6 heavy chain variable region gene families. Mol. Immunol. 30: 1601–1610.
Adderson EE, Azmi FH, Wilson PM, Shackelford PG, Carroll WL. (1993) The human VH3b gene subfamily is highly polymorphic. J. Immunol. 151: 800–809.
Berman JE, Mellis SJ, Pollock R, et al. (1988) Content and organization of the human Ig VH locus: Definition of three new VH families and linkage to the Ig CH locus. E.M.B.O. J. 7: 727–738.
Hieter PA, Maizel JV, Leder P. (1982) Evolution of human immunoglobulin kappa J region genes. J. Biol. Chem. 257: 1516–1522.
Anker R, Caldwell J, Brokaw J, Pollok BA. (1989) Characterization of immunoglobulin mRNA expression in Burkitt lymphoma cell lines. Int. J. Cancer 43: 930–935.
Klein R, Jaenichen R, Zachau HG. (1993) Expressed human immunoglobulin κ genes and their hypermutation. Eur. J. Immunol. 23: 3248–3271.
Cogne M, Mounir S, Mahdi T, Preud’homme JL, Guglielmi P. (1990) Production of an abnormal μ chain with a shortened VHIV subgroup variable region in a Burkitt’s lymphoma cell line. Mol. Immunol. 27: 929–934.
Cogne M, Mounir S, Aucouturier P, Preud’homme JL, Nau F, Guglielmi P. (1990) Immunoglobulin light chain transcripts with altered V regions in Burkitt’s lymphoma cell lines producing short μ chains. Eur. J. Immunol. 20: 1905–1910.
Jäck H, Berg J, Wabl M. (1989) Translation affects immunoglobulin mRNA stability. Eur. J. Immol. 19: 843–847.
Rowe M, Rooney CM, Edwards CF, Lenoir GM, Rickinson AB. (1986) Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt’s lymphoma. Int. J. Cancer 37: 367–373.
Liu YJ, Johnson GD, Gordon J, MacLennan ICM. (1992) Germinal centres in T-cell-dependent antibody responses. Immunol. Today 13: 17–21.
Logtenberg T, Schutte ME, Ebeling SB, Gmelig-Meyling FHJ, van Es JH. (1992) Molecular approaches to the study of human B-cell and (auto) antibody repertoire generation and selection. Immunol. Rev. 128: 23–47.
Reynaud CA, Garcia C, Hein WR, Weill JC. (1995) Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell 80: 115–125.
Reynaud CA, Mackay CR, Müller RG, Weill JC. (1991) Somatic generation of diversity in a mammalian primary lymphoid organ: The sheep ileal Peyer’s patches. Cell 64: 995–1005.
Weber JC, Blaison G, Martin T, Knapp AM, Pasquali JL. (1994) Evidence that the VkIII gene usage is nonstochastic in both adult and newborn peripheral B cells and that peripheral CD5+ adult B cells are oligoclonal. J. Clin. Invest. 93: 2093–2105.
Cleary ML, Meeker TC, Levy S, et al. (1986) Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell 44: 97–106.
Bahler DW, Levy R. (1992) Clonal evolution of a follicular lymphoma: Evidence for antigen selection. Proc. Natl. Acad. Sci. U.S.A. 89: 6770–6774.
Chapman CJ, Mockridge CI, Rowe M, Rickinson AB, Stevenson FK. (1995) Analysis of VH genes used by neoplastic B cells in endemic Burkitt’s lymphoma shows somatic hypermutation and intraclonal heterogeneity. Blood 85: 2176–2181.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft through Di184 and SFB 242. We thank H. G. Zachau for helpful discussions and H. Gustav Klobeck for critical reading of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klein, U., Klein, G., Ehlin-Henriksson, B. et al. Burkitt’s Lymphoma Is a Malignancy of Mature B Cells Expressing Somatically Mutated V Region Genes. Mol Med 1, 495–505 (1995). https://doi.org/10.1007/BF03401587
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401587