Abstract
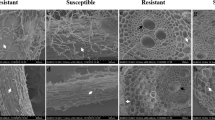
Electron microscopy studies were carried out to investigate the association of visible disease symptoms with ultrastructural changes in barley plants infected with mild and aggressive isolates of Barley stripe mosaic virus (BSMV). Differences in virus particle concentrations and cytopathological alterations in leaves were observed. The formation of membranous inclusions, abnormal dumbbell-shaped mitochondria and the deposition of membrane structures between the cell wall and plasma membrane were characteristic for aggressive isolates, whereas the rearrangement of the thylakoids and appearing electron transparent vacuoles in the plastid stroma were typical for the mild isolate. Moreover, the invaginations of plasma membrane into the cell and the cytoplasm containing a large number of virus particles into the vacuole, elongated mitochondria and alteration of micro-bodies were observed in the case of both isolates.
Similar content being viewed by others
References
Almási A, Apatini D & Bóka K, Böddi B & Gáborjányi R, 2000. BSMV infection inhibits chlorophyll biosynthesis in barley plants. Physiol Mol Plant Pathol 56, 227–233.
Barker H & Harrison BD, 1976. Infection of Tobacco mesophyll protoplasts with Raspberry ringspot virus alone and together with Tobacco rattle virus. J Gen Virol 35,125–133.
Bassi M, Appiano A, Barbieri N & D’Agosto G, 1985. Chloroplast alterations induces by Tomato bushy stunt virus in datura leaves. Protoplasma 126, 233–235.
Bragg JN & Jackson AO, 2004. Barley stripe mosaic. In Lapierre, H & Signoret, PA (Ed.) 2004: Viruses and Virus Diseases of Poaceae (Gramineae). INRA, Paris. 456–457.
Brunt AA, Crabtree K, Dallwitz MJ, Gibbs AJ, Watson L & Zurcher EJ, 1996. Plant Viruses Online: Descriptions and Lists from the VIDE Database. Article first published online: 20 AUG 1996 | URL http://biology.anu.edu.au/Groups/MES/vide/.
Carroll TW, 1969. Electron microscopic evidence for the presence of Barley stripe mosaic virus in cells of barley embryos. Virology 37, 649–657.
Carroll TW, 1970. Relation of Barley stripe mosaic virus to plastids. Virology 42, 1015–1022.
Carroll TW, 1974. Barley stripe mosaic virus in sperm and vegetative cells of barley pollen. Virology 60, 21–28.
Carroll TW, 1986. Hordeiviruses, Biology and Pathology. In: Van Regenmortel, MHV & Fraenkel-Conrat, H (Ed.) 1986: The Plant Viruses Volume 2. The rod-shaped plant viruses. Plenum Press, New York and London. 373–395.
Cass DD & Jensen WA, 1970. Fertilization in barley. Amer J Bot 57, 62–70.
Chong J & Haber S, 1992. Cytological alterations associated with flame chlorosis, a novel viruslike disease of barley, wheat, and oat. Phytopathology 82, 815–821.
Clark MF & Adams AN, 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34, 475–483.
Francki RIB, Milne RG & Hatta T, 1984. Atlas of plant viruses, Volume II. CRC Press., Boca Raton, USA.
Gao R, Liu P & Wong S-M, 2012. Identification of plant viral RNA genome in the nucleus. Plos One 7, e48736.
Gardner WS, 1967. Electron microscopy of Barley stripe mosaic virus: comparative cytology of tissues infected during different stages of maturity. Phytopathology 57, 1315–1326.
Harsányi A, Böddi B & Bóka K, Almási A & Gáborjányi R, 2002. Abnormal etioplast development in barley seedlings infected with BSMV by seed transmission. Physiol Plantarum 114, 149–155.
Hibino H, Esuchizaki T & Saito Y, 1974. Comparative electron microscopy inclusions induced by 9 isolates of Soil-born wheat mosaic virus. Virology 57, 510–521.
Hoefert LL, Esau K & Duffus JE, 1970. Electron microscopy of beta leaves infected with Beet yellow stunt virus. Virology 42, 814–824.
Honda Y & Matsui C, 1971. Electron microscopy of intracellular Radish mosaic virus. Phytopathol 62, 448–452.
Jackson AO, Petty ITD, Jones RW, Edwards MC & French R, 1991. Analysis of barley stripe mosaic virus pathogenicity. Sem Virol 2, 107–119.
Jeżewska M, 2001. Identification of Barley stripe mosaic virus in Poland. J Plant Prot Res 41, 164–167.
Jeżewska M, 2006. Wirusy zbóz przenoszone przez nasiona-występowanie w Polsce i potencjalna szkodliwość. [Seedtransmitted cereal viruses-occurrence in Poland and their potential harmfulness]. Habilitation dissertation. Rozprawy Naukowe Instytutu Ochrony Roslin, Zeszyt 14. ISSN 1730-038X.
Kendall A, Williams D, Bian W, Stewart PL & Stubbs G, 2013. Barley stripe mosaic virus: structure and relationship to the tobamoviruses. Virology 443, 265–270.
Koenig R, An D, Lesemann De & Burgermeister W, 1987. Isolation of Carnation ringspot virus from canal near a sewage plant: cDNA hybridization analysis, serology and cytopathology. J Phytopathol 121, 346–356.
Lin NS & Langenberg WG, 2004. Chronology of appearance of Barley stripe mosaic virus protein infected wheat cells. J Ultra Res 89, 309–323.
Martelli G & Russo M, 1976. Unusual cytoplasmic inclusions induced by Watermelon mosaic virus. Virology 72, 352–362.
McMullen CR, Gardner WS & Myers GA, 1977. Ultrastructure of cell wall thickenings and paramural bodies induced by Barley stripe mosaic virus. Phytopathology 67, 462–467.
McMullen CR, Gardner WS & Myers GA, 1978. Aberrant plastids in barley leaf tissue infected with Barley stripe mosaic virus. Phytopathology 68, 317–325.
Rana GL, Franco AD, Piazzolla P & Migliori A, 1987. Further studies on a Tomato ring virus isolate from Artichoke. J Phytopathol 118, 203–211.
Russo M & Martelli GP, 1972. Ultrastructural observations of Tomato bushy stunt virus in plant cells. Virology 49, 122–129.
Shalla TA, 1959. Relations of Tobacco mosaic virus and Barley stripe mosaic virus to their host cells as revealed by ultrathin tissue-sectioning for the electron microscope. Virology 7, 193–219.
Stewart AD, Logsdon JM & Kelley ST, 2005. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59, 730–73.
Torrance L, Cowan GH, Gillespie T, Ziegler A & Lacomme C, 2006. Barley stripe mosaic virus-encoded proteins triple-gene block 2 and γb localize to chloroplasts in virus-infected monocot and dicot plants, revealing hitherto-unknown roles in virus replication. J Gen Virol 87, 2403–2411.
Weintraub M & Ragetli HWJ, 1970. Electron microscopy of the bean and cowpea strains of Southern bean mosaic virus within leaf cells. J Ultra Res 32, 167–189.
Zarzyńska A, Jeżewska M, Trzmiel K & Hasiów-Jaroszerwska B, 2014. Development of a one-step immunocapture realtime RT-PCR assay for the detection of Barley stripe mosaic virus strains in barley seedlings. Acta Virol 58, 81–85.
Zielińska L & Pospieszny H, 2002. Ultrastructural changes in leaf cells infected with Arabis mosaic nepovirus II. in Chenopodium quinoa plants. J Plant Protect Res 42, 189–198.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarzyńska-Nowak, A., Jeżewska, M., Hasiów-Jaroszewska, B. et al. A Comparison of Ultrastructural Changes of Barley Cells Infected with Mild and Aggressive Isolates of Barley stripe mosaic virus. J Plant Dis Prot 122, 153–160 (2015). https://doi.org/10.1007/BF03356545
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03356545