Abstract
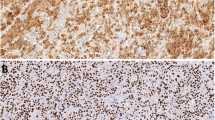
An altered control of the mechanisms involved in cell proliferation and programmed cell death (apoptosis) might play an important role in parathyroid tumorigenesis. We evaluated by immunohistochemistry the expression of bcl-2 and p53 proteins, as markers of apoptosis control, and MIB-1, as marker of cell proliferation, in a series of normal and neoplastic parathyroid tissues. The specimens were 33 normal parathyroids, 43 parathyroid adenomas and 3 parathyroid carcinomas. Results were scored as positive when more than 1% of cells were stained for MIB-1 and p53, and more than 10% for bcl-2. All normal parathyroids showed numerous bcl-2 positive cells (≥80%), low proliferation rate (MIB-1) and no p53 protein expression. Twenty-four (55%) adenomas were bcl-2 positive; in 16 of these the number of positive cells was high (>50%) and immunoreactivity was diffusely distributed within the adenoma; 8 cases showed a zonal staining pattern, in which groups of stained cells were surrounded by negative cells. Nineteen adenomas (45%) and all carcinomas were bcl-2 negative. A high proliferative rate (MIB-1) was found in all carcinomas and 4 adenomas (9%); all MIB-1 positive adenomas were bcl-2 negative. p53 was negative in all specimens. No significant differences in serum calcium and intact PTH levels nor in tumor size were found between bcl-2 negative and bcl-2-positive and MIB-1-positive and MIB-1-negative adenomas. An inverse, but not statistically significant (p=0.06) correlation was observed between the percentage of bcl-2 positive cells and serum calcium level in parathyroid adenomas. In conclusion, parathyroid adenomas are a heterogeneous group of lesions in which the pattern of bcl-2 and MIB-1 protein expression ranges between that of normal parathyroid (bcl-2 positivity and MIB-1 negativity) and that of parathyroid carcinoma (bcl-2 negativity and MIB-1 positivity). The question of whether the finding of the MIB-1 positive-bcl-2 negative phenotype identifies a subgroup of clinically more aggressive adenomas remains to be established.
Similar content being viewed by others
References
Cattoretti G., Becker M.H.G., Key G., Ouchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant part of the ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in micro-wave processed formalin-fixed paraffin sections. J. Pathol. 168: 357, 1992.
Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab V., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133: 1710, 1984.
Hockenbery D.M., Zutter M., Hickey W., Nahm M., Korsmeyer S.J. Bcl2 protein is topographically restricted in tissue characterized by apoptotic cell death. Proc. Natl. Acad. Sci. USA 88: 6961, 1991.
Stewart B.W. Mechanism of apoptosis: integration of genetic, biochemical and cellular indicators. J. Natl. Cancer Inst. 86: 1286, 1994.
Lowe S.W., Schmitt E.M., Smith S.W., Osborne B.A., Jack T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847, 1993.
Hockenbery D., Oltvai Z., Yin X-M., Milliman C., Korsmeyer S.J. bcl-2 functions in an antioxidant pathway of apoptosis. Cell 75: 241, 1993.
Lotem J., Sachs L. Regulation by bcl-2, c-myc and p53 of susceptibility to induction of apoptosis by heat shock and cancer chemotherapy compounds in differentiation-competent and -defective myeloid leukemic cells. Cell Growth Differ. 4: 41, 1993.
Ginsberg D., Mechta F., Yaniv M., Otem M. Wild-type p53 can down modulate the activity of various promoters. Proc. Natl. Acad. Sci Usa 88: 9979, 1991.
Abbona G.C., Papotti M., Gasparri G., Bussolati G. Proliferative activity in parathyroid tumors as detected by Ki-67 immunostaining. Hum. Pathol. 26: 135, 1995.
Vargas M.P., Vargas H.I., Kleine D.E., Merino M.J. The role of prognostic markers (MiB-1, RB, and bcl-2) in the diagnosis of parathyroid tumors. Mod. Pathol. 10: 12, 1997.
Cryns V.L., Rubio M.P., Thor A.D., Louis D.N., Arnold A. p53 abnormalities in human parathyroid carcinomas. J. Clin. Endocrinol. Metab. 78: 1320, 1994.
Cordeil J.L., Falini B., Erber W.N., Ghosh A.K., Abdul-Aziz Z., MacDonald S., Pulford K.A.F., Stein H., Mason D. Immunoenzymatic labelling of monoclonal antibody using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J. Histochem. Cytochem. 32: 219, 1984.
Hockenbery D.M., Nunez G., Milliman C., Schreiber R.D., Korsmeyer S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334, 1990.
Lu Q-L., Abel P., Foster C.F., Lalani E-N. bcl-2: role in epithelial differentiation and oncogenesis. Hum. Pathol. 27: 102, 1996.
Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Orem M. Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature 352, 345–347, 1991.
Hall P.A., Woods A.L. Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet. 23: 505, 1990.
Berland Y., Olmer M., Lebreuil G., Grisoli J. Parathyroid carcinoma, adenoma and hyperplasia in a case of chronic renal insufficiency on dialysis. Clin. Nephrol. 18: 154, 1982.
Desch C.E., Arsensis G., May A.G., Amatruda J.M. Parathyroid hyperplasia and carcinoma within one gland. Am. J. Med. 77: 131, 1984.
Arnold A., Staunton C.E., Kim H.G., Gaz R.D., Kronenberg H.M. Monoclonality and abnormal parathyroid hormon genes in parathyroid adenomas. N. Engl. J. Med. 318: 658, 1988.
Schmid K.W., Hittmair A., Ladurner D., Sandbichler P., Gasser R., Tötsch M. Chromogranin A and B parathyroid tissue of cases of primary hyperparathyroidism: an immunohistochemical study. Virchows Arch. A Pathol. Anat. 418: 295, 1991.
Fain R.P., Kort N.E., Yousry C., James R.M., Litt M. A high resolution CEPH crossover mapping panel and integrated map of chromosome 11. Hum. Mol. Genet. 5: 1631, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Naccarato, A.G., Marcocci, C., Miccoli, P. et al. Bcl-2, p53 and MIB-1 expression in normal and neoplastic parathyroid tissues. J Endocrinol Invest 21, 136–141 (1998). https://doi.org/10.1007/BF03347291
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347291