Abstract
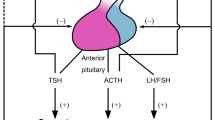
Depression is one of the most common psychiatric disorders. For a long time, clinicians suspected a causal link between depression and the endocrine system. The most frequently occurring endocrine abnormality in depressed subjects is hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis. CRH and AVP are likely to play a substantial role in the pathophysiology of this disorder, and their receptors appear to be a specific target for future antidepressant drugs. Depression also affects the hypothalamic-pituitary-GH (HPGH) and -thyroid (HPT) axes. Alterations in the reproductive system may also play a role in the pathology of depression. In addition, there is increasing evidence that leptin and neurosteroids, such as DHEA, are implicated in mood disorders.
Similar content being viewed by others
References
Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994, 51: 8–19.
Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci 2002, 5 (Suppl): 1068–70.
Wittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1994, 51: 355–64.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349: 1498–504.
Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998, 55: 580–92.
Shapiro PA, Lesperance F, Frasure-Smith N, et al. An open-label preliminary trial of sertraline for treatment of major depression after acute myocardial infarction (the SADHAT Trial). Sertraline Anti-Depressant Heart Attack Trial. Am Heart J 1999, 137: 1100–6.
Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol 1995, 76: 562–4.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23: 477–501.
Lasser RA, Baldessarini RJ. Thyroid hormones in depressive disorders: a reappraisal of clinical utility. Harv Rev Psychiatry 1997, 4: 291–305.
Dinan TG. Psychoneuroendocrinology of depression. Growth hormone. Psychiatr Clin North Am 1998, 21: 325–39.
Young EA, Korszun A. The hypothalamic-pituitary-gonadal axis in mood disorders. Endocrinol Metab Clin North Am 2002, 31: 63–78.
Holsboer F, Barden N. Antidepressants and hypothalamicpituitary-adrenocortical regulation. Endocr Rev 1996, 17: 187–205.
Scott LV, Dinan TG. Vasopressin as a target for antidepressant development: an assessment of the available evidence. J Affect Disord 2002, 72: 113–24.
Linkowski P, Mendlewicz J, Leclercq R, et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab 1985, 61: 429–38.
Mortola JF, Liu JH, Gillin JC, Rasmussen DD, Yen SS. Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab 1987, 65: 962–8.
Deuschle M, Schweiger U, Weber B et, al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab 1997, 82: 234–8.
Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984, 226: 1342–4.
Nemeroff CB, Bissette G, Akil H, Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry 1991, 158:59–63: 59–63.
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 1994, 60: 436–44.
Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988, 45: 577–9.
Krishnan KR, Doraiswamy PM, Lurie SN, et al. Pituitary size in depression. J Clin Endocrinol Metab 1991, 72: 256–9.
Axelson DA, Doraiswamy PM, Boyko OB, et al. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patients. Psychiatry Res 1992, 44: 63–70.
Nemeroff CB, Krishnan KR, Reed D, Leder R, Beam C, Dunnick NR. Adrenal gland enlargement in major depression. A computed tomographic study. Arch Gen Psychiatry 1992, 49(5):384–7.
Rubin RT, Phillips JJ, Sadow TF, McCracken JT. Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. Arch Gen Psychiatry 1995, 52: 213–8.
Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 2001, 62: 77–91.
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999, 160: 1–12.
Gold PW, Loriaux DL, Roy A,et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med 1986, 314: 1329–35.
Holsboer F, Gerken A, von Bardeleben U, et al. Human corticotropin-releasing hormone in depression—correlation with thyrotropin secretion following thyrotropin-releasing hormone. Biol Psychiatry 1986, 21: 601–11.
von Bardeleben U, Holsboer F. Effect of age on the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry 1991, 29: 1042–50.
Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 1994 28: 341–56.
Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J Psychiatr Res 1999, 33: 363–70.
Kunzel HE, Binder EB, Nickel T, et al. Pharmacological and nonpharmacological factors influencing hypothalamic-pituitary-adrenocortical axis reactivity in acutely depressed psychiatric in-patients, measured by the Dex-CRH test. Neuropsychopharmacology 2003, 28: 2169–78.
Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P- Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 1995, 96: 1698–705.
Uhr M, Holsboer F, Muller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 2002, 14: 753–9.
Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, De Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology 1998, 139: 1789–93.
van Haarst AD, Oitzl MS, Workel JO, De Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology 1996, 137: 4935–43.
Keck ME, Wigger A, Welt T, et al. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychopharmacology 2002, 26: 94–105.
Holsboer F. Corticotropin-releasing hormone modulators and depression. Curr Opin Investig Drugs 2003, 4: 46–50.
Muller M, Holsboer F, Keck ME. Genetic modification of corticosteroid receptor signalling: novel insights into pathophysiology and treatment strategies of human affective disorders. Neuropeptides 2002, 36: 117–31.
Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997, 20: 78–84.
Lovenberg TW, Liaw CW, Grigoriadis DE, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A 1995, 92: 836–40.
Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci 1996, 17: 166–72.
Grigoriadis DE, Lovenberg TW, Chalmers DT, Liaw C, De Souze EB. Characterization of corticotropin-releasing factor receptor subtypes. Ann N Y Acad Sci 1996, 780: 60–80.
Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2002, 2: 23–33.
Schulkin J, Gold PW, McEwen BS. Induction of corticotropin- releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 1998, 23: 219–43.
Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol 1996, 17: 281–326.
Joels M, De Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol 1994, 43: 1–36.
De Kloet ER, Sutanto W, Rots N, et al. Plasticity and function of brain corticosteroid receptors during aging. Acta Endocrinol (Copenh) 1991, 125 (Suppl) 1: 65–72.
Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 2002, 73: 147–58.
Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid- receptor function in mice bearing antisense RNA transgene. Nature 1992, 355: 725–8.
Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol 2000, 20: 9009–17.
Cole TJ, Blendy JA, Monaghan AP, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 1995, 9: 1608–21.
Tronche F, Kellendonk C, Kretz O, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999, 23: 99–103.
Berger S, Bleich M, Schmid W, et al. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A 1998, 95: 9424–9.
Gass P, Kretz O, Wolfer DP, et al. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep 2000, 1: 447–51.
Vaughan J, Donaldson C, Bittencourt J et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 1995, 378: 287–92.
Reyes TM, Lewis K, Perrin MH et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A 2001, 98: 2843–8.
Lewis K, Li C, Perrin MH et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A 2001, 98: 7570–5.
Hsu SY, Hsueh AJ. Human stresscopin and stresscopinrelated peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med 2001, 7: 605–11.
Liebsch G, Landgraf R, Engelmann M, Lorscher P, Holsboer F. Differential behavioural effects of chronic infusion of CRH 1 and CRH 2 receptor antisense oligonucleotides into the rat brain. J Psychiatr Res 1999, 33(2):153–63.
Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 1998, 19: 162–6.
Keck ME, Welt T, Wigger A, et al. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci 2001, 13: 373–80.
Lancel M, Muller-Preuss P, Wigger A, Landgraf R, Holsboer F. The CRH1 receptor antagonist R121919 attenuates stress-elicited sleep disturbances in rats, particularly in those with high innate anxiety. J Psychiatr Res 2002, 36: 197–208.
Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology 2002, 27: 194–202.
Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther 2003, 304: 874–80.
Keck ME, Welt T, Muller MB, Landgraf R, Holsboer F. The high-affinity non-peptide CRH1 receptor antagonist R121919 attenuates stress-induced alterations in plasma oxytocin, prolactin, and testosterone secretion in rats. Pharmacopsychiatry 2003, 36: 27–31.
Kunzel HE, Zobel AW, Nickel T, et al. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res 2003, 37: 525–33.
Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 2001, 22: 835–44.
Zobel AW, Nickel T, Kunzel HE, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000, 34: 171–81.
Muller MB, Zimmermann S, Sillaber I, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxietyrelated behavior and hormonal adaptation to stress. Nat Neurosci 2003, 6: 1100–7.
Antoni FA. Vasopressinergic control of pituitary adreno-corticotropin secretion comes of age. Front Neuroendocrinol 1993, 14: 76–122.
Keck ME, Sillaber I, Ebner K, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci 2000, 12: 3713–20.
Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 1996, 53: 137–43.
van Londen L, Goekoop JG, van Kempen GM, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 1997, 17: 284–92.
von Bardeleben U, Holsboer F, Stalla GK, Muller OA. Combined administration of human corticotropin-releasing factor and lysine vasopressin induces cortisol escape from dexamethasone suppression in healthy subjects. Life Sci 1985, 37: 1613–8.
Griebel G, Simiand J, Serradeil-Le Gal C, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A 2002, 99: 6370–5.
Landgraf R, Gerstberger R, Montkowski A et al. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci 1995, 15: 4250–8.
Keck ME, Welt T, Muller MB et al. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology 2003, 28: 235–43.
Zhou JN, Riemersma RF, Unmehopa UA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry 2001, 58: 655–62.
Steiger A, von Bardeleben U, Herth T, Holsboer F. Sleep EEG and nocturnal secretion of cortisol and growth hormone in male patients with endogenous depression before treatment and after recovery. J Affect Disord 1989, 16: 189–95.
Jarrett DB, Miewald JM, Kupfer DJ. Recurrent depression is associated with a persistent reduction in sleep-related growth hormone secretion. Arch Gen Psychiatry 1990, 47: 113–8.
Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression. X: Serum growth hormone measures in patients and matched control subjects. Biol Psychiatry 1990, 27: 1065–82.
Voderholzer U, Laakmann G, Wittmann R, et al. Profiles of spontaneous 24-hour and stimulated growth hormone secretion in male patients with endogenous depression. Psychiatry Res 1993, 47: 215–27.
Sakkas PN, Soldatos CR, Bergiannaki JD, Paparrigopoulos TJ, Stefanis CN. Growth hormone secretion during sleep in male depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 1998, 22: 467–83.
Gerner RH, Yamada T. Altered neuropeptide concentrations in cerebrospinal fluid of psychiatric patients. Brain Res 1982, 238: 298–302.
Rubinow DR. Cerebrospinal fluid somatostatin and psychiatric illness. Biol Psychiatry 1986, 21: 341–65.
Doran AR, Rubinow DR, Roy A, Pickar D. CSF somatostatin and abnormal response to dexamethasone administration in schizophrenic and depressed patients. Arch Gen Psychiatry 1986, 43: 365–9.
Chappell PB, Smith MA, Kilts CD, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 1986, 6: 2908–14.
Sunderland T, Rubinow DR, Tariot PN, et al. CSF somatostatin in patients with Alzheimer’s disease, older depressed patients, and age-matched control subjects. Am J Psychiatry 1987, 144: 1313–6.
Molchan SE, Lawlor BA, Hill JL, et al. CSF monoamine metabolites and somatostatin in Alzheimer’s disease and major depression. Biol Psychiatry 1991, 29: 1110–8.
Heuser I, Bissette G, Dettling M, et al. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 1998, 8(2):71–9.
Matussek N, Ackenheil M, Hippius H, et al. Effect of clonidine on growth hormone release in psychiatric patients and controls. Psychiatry Res 1980, 2: 25–36.
Checkley SA, Glass IB, Thompson C, Corn T, Robinson P. The GH response to clonidine in endogenous as compared with reactive depression. Psychol Med 1984, 14: 773–7.
Siever LJ, Uhde TW, Silberman EK, et al. Growth hormone response to clonidine as a probe of noradrenergic receptor responsiveness in affective disorder patients and controls. Psychiatry Res 1982, 6: 171–83.
Mokrani M, Duval F, Diep TS, Bailey PE, Macher JP. Multihormonal responses to clonidine in patients with affective and psychotic symptoms. Psychoneuroendocrinology 2000, 25: 741–52.
Ryan ND, Dahl RE, Birmaher B, et al. Stimulatory tests of growth hormone secretion in prepubertal major depression: depressed versus normal children. J Am Acad Child Adolesc Psychiatry 1994, 33: 824–33.
Dahl RE, Birmaher B, Williamson DE et al. Low growth hormone response to growth hormone-releasing hormone in child depression. Biol Psychiatry 2000, 48: 981–8.
Birmaher B, Dahl RE, Williamson DE, et al. Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Arch Gen Psychiatry 2000, 57: 867–72.
Lesch KP, Rupprecht R, Muller U, Pfuller H, Beckmann H. Insulin-like growth factor I in depressed patients and controls. Acta Psychiatr Scand 1988, 78(: 684–8.
Rupprecht R, Lesch KP. Psychoneuroendocrine research in depression. I. Hormone levels of different neuroendocrine axes and the dexamethasone suppression test. J Neural Transm 1989, 75(3):167–78.
Deuschle M, Blum WF, Strasburger CJ, et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology 1997, 22: 493–503.
Franz B, Buysse DJ, Cherry CR, et al. Insulin-like growth factor 1 and growth hormone binding protein in depression: a preliminary communication. J Psychiatr Res 1999, 33: 121–7.
Rivier C, Vale W. Involvement of corticotropin-releasing factor and somatostatin in stress-induced inhibition of growth hormone secretion in the rat. Endocrinology 1985, 117: 2478–82.
Barbarino A, Corsello SM, Della CS, et al. Corticotropin-releasing hormone inhibition of growth hormone-releasing hormone-induced growth hormone release in man. J Clin Endocrinol Metab 1990, 71: 1368–74.
Holsboer F, von Bardeleben U, Steiger A. Effects of intravenous corticotropin-releasing hormone upon sleeprelated growth hormone surge and sleep EEG in man. Neuroendocrinology 1988, 48: 32–8.
Wiedemann K, von Bardeleben U, Holsboer F. Influence of human corticotropin-releasing hormone and adrenocorticotropin upon spontaneous growth hormone secretion. Neuroendocrinology 1991, 54: 462–8.
Vandoolaeghe E, Maes M, Vandevyvere J, Neels H. Hypothalamic-pituitary-thyroid-axis function in treatment resistant depression. J Affect Disord 1997, 43: 143–50.
Bartalena L, Placidi GF, Martino E, et al. Nocturnal serum thyrotropin (TSH) surge and the TSH response to TSHreleasing hormone: dissociated behavior in untreated depressives. J Clin Endocrinol Metab 1990, 71: 650–5.
Holsboer F, Gerken A, von Bardeleben U, Grimm W, Stalla GK, Muller OA. Relationship between pituitary responses to human corticotropin-releasing factor and thyrotropinreleasing hormone in depressives and normal controls. Eur J Pharmacol 1985, 110: 153–4.
Kendler KS, Davis KL. Elevated corticosteroids as a possible cause of abnormal neuroendocrine function in depressive illness. Commun Psychopharmacol 1977, 1: 183–94.
Marangell LB, George MS, Callahan AM, et al. Effects of intrathecal thyrotropin-releasing hormone (protirelin) in refractory depressed patients. Arch Gen Psychiatry 1997, 54: 214–22.
Callahan AM, Frye MA, Marangell LB, et al. Comparative antidepressant effects of intravenous and intrathecal thyrotropin-releasing hormone: confounding effects of tolerance and implications for therapeutics. Biol Psychiatry 1997, 41: 264–72.
Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001, 158: 1617–22.
Pinna G, Broedel O, Eravci M, et al. Thyroid hormones in the rat amygdala as common targets for antidepressant drugs, mood stabilizers, and sleep deprivation. Biol Psychiatry 2003, 54: 1049–59.
Fountoulakis KN, Iacovides A, Grammaticos P, St Kaprinis G, Bech P. Thyroid function in clinical subtypes of major depression: an exploratory study. BMC Psychiatry 2004, 4: 6–1.
Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry 1977, 34: 98–111.
Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry 1993, 5: 249–54.
MacLusky NJ, Naftolin F, Leranth C. Immunocyto-chemical evidence for direct synaptic connections between corticotrophin-releasing factor (CRF) and gonadotrophin-releasing hormone (GnRH)-containing neurons in the preoptic area of the rat. Brain Res 1988, 439: 391–5.
Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999, 61: 292–6.
Steiger A, von Bardeleben U, Wiedemann K, Holsboer F. Sleep EEG and nocturnal secretion of testosterone and cortisol in patients with major endogenous depression during acute phase and after remission. J Psychiatr Res 1991, 25: 169–77.
Pope HG, Jr., Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003, 160: 105–11.
Attardi B, Tsujii T, Friedman R, et al. Glucocorticoid repression of gonadotropin-releasing hormone gene expression and secretion in morphologically distinct subpopulations of GT1-7 cells. Mol Cell Endocrinol 1997, 131(2): 241–55.
Chandran UR, Attardi B, Friedman R, Dong KW, Roberts JL, DeFranco DB. Glucocorticoid receptor-mediated repression of gonadotropin-releasing hormone promoter activity in GT1 hypothalamic cell lines. Endocrinology 1994, 134: 1467–74.
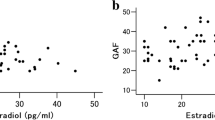
Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the harvard study of moods and cycles. Arch Gen Psychiatry 2003, 60: 29–36.
Van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003, 165: 97–110.
Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003, 28: 139–68.
Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62: 265–71.
Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoidlike effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 1996, 15: 533–40.
Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav 2000, 67: 137–43.
Strohle A, Pasini A, Romeo E, et al. Fluoxetine decreases concentrations of 3 alpha, 5 alpha-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res 2000, 34: 183–6.
Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc 1999, 47 685–91.
Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J Clin Endocrinol Metab 1998, 83: 3130–3.
Takebayashi M, Kagaya A, Uchitomi Y, et al. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm 1998, 105: 537–42.
Fabian TJ, Dew MA, Pollock BG, et al. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry 2001, 50: 767–74.
Osran H, Reist C, Chen CC, Lifrak ET, Chicz-DeMet A, Parker LN. Adrenal androgens and cortisol in major depression. Am J Psychiatry 1993, 150(5):806–9.
Scott LV, Salahuddin F, Cooney J, Svec F, Dinan TG. Differences in adrenal steroid profile in chronic fatigue syndrome, in depression and in health. J Affect Disord 1999, 54: 129–37.
Wolkowitz OM, Reus VI, Roberts E, et al. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry 1997, 41: 311–8.
Wolkowitz OM, Reus VI, Keebler A, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 1999, 156: 646–9.
Schwartz MW. Regulation of appetite and body weight. Hosp Pract (Off Ed) 1997, 32: 109.
Considine RV, Sinha MK, Heiman ML et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996, 334: 292–5.
Pollmacher T. [Leptin and psychiatric disorders]. Nervenarzt 2002, 73: 897–902.
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitaryadrenal axis in response to stress. Endocrinology 1997, 138: 3859–63.
Deuschle M, Blum WF, Englaro P, et al. Plasma leptin in depressed patients and healthy controls. Horm Metab Res 1996, 28: 714–7.
Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res 1998, 32: 403–10.
Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology 2001, 73: 243–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tichomirowa, M.A., Keck, M.E., Schneider, H.J. et al. Endocrine disturbances in depression. J Endocrinol Invest 28, 89–99 (2005). https://doi.org/10.1007/BF03345535
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03345535